A summary of Pinheiro, I., et al (2017). A yeast fermentate improves gastrointestinal discomfort and constipation by modulation of the gut microbiome: results from a randomized double-blind placebo-controlled pilot trial. BMC Complement Altern Med 17 (1), 441.
Summary
The aim of the study was to investigate the effects of yeast fermentate on gut health in a healthy population with symptoms of gastrointestinal (GI) discomfort and reduced bowel movements.1 This study helped to confirm previous published in vitro and animal studies demonstrating yeast fermentate’s gastrointestinal and prebiotic benefits at only 500 mg dose.2-4 Results include:
- Statistically significant reduction in gastrointestinal discomfort compared to placebo.
- Statistically significant improvement in stool consistency compared to placebo.
- Statistically significant decrease in Firmicutes with a relative increase in Bacteroidetes when compared to placebo which leads to a decrease the Firmicutes/Bacterioetes (F/B) ratio.
- Statistically significant increase in Bacteroides and Prevotella when compared to placebo may link yeast fermentate’s microbiome modulation to improved consistency and frequency of stools.
- Statistically significant improvement in Quality of Life validated questionnaire found within yeast fermentate group, but not in placebo group include:
- Physical Discomfort
- Psychosocial Discomfort
- Satisfaction
- Significant improvement in Perceived Stress validated questionnaire within both the yeast fermentate total cohort and severe subgroup, but not within the placebo groups.
- Strong trend for improvement in stool frequency compared to placebo.
Introduction
Constipation and symptoms of gastrointestinal (GI) discomfort, such as bloating, are common among otherwise healthy individuals. Despite the recognized contribution of the gut microbiome to this pathology, little is known about which groups of microorganisms play a role in GI discomfort.
The bulk of current clinical science performed on yeast fermentate showed significant immune health benefits.5-10 It was postulated that these immune benefits occur primarily through yeast fermentate ’s interaction with the gut. In fact, an in vitro study indicated that yeast fermentate has prebiotic-like properties by increasing total Short Chain Fatty Acids (SCFAs), while shifting the pattern of SCFA composition to produce more butyrate.2 Further experiments revealed that yeast fermentate modified the total gut flora and affected what type of bacteria adhered to the simulated gut wall.3 There was a trend for increased adherence of Lactobacilli species and a trend for decreased adherence of Clostridia species.3
In addition, animal models showed yeast fermentate maintained integrity of healthy gut morphology in terms of villus height and total mucosal thickness when the animals were exposed to heat stress.4 The yeast fermentate treated animals also had reduced lipopolysaccharide (LPS) endotoxin leakage from the gut into the circulatory system.4
Those results prompted the study summarized here: a human clinical trial on healthy subjects with mild constipation symptoms. The primary endpoints included constipation symptoms such as frequency, consistency and GI discomfort, while secondary endpoints included modulation of the gut microbiome.
Methods
Using a double-blinded, placebo-controlled parallel design, 80 healthy subjects with symptoms of gastrointestinal discomfort and constipation were equally allocated to one of two trial arms: placebo or 500 mg yeast fermentate. There was a two-week run-in phase followed by a six-week intervention phase. Randomization was done in a stratified manner according symptom severity, resulting in two subgroups of patients: severe (n=55) and moderate (n=25). Results are given for the total cohort as well as the severe and moderate subgroups.
Primary Endpoints
GI discomfort and quality of life parameters were measured using three different instruments: a GI symptoms diary and two validated quality of life questionnaires, the Patient Assessment of Constipation Quality of Life (PAC-QOL) and Perceived Stress Scale (PSS) questionnaires.
Secondary Endpoints
Qualitative changes in the microbiome of the gut were assayed using fecal samples that were collected at baseline and at three and six weeks. The general microbiota structure and profiles found in the samples was determined by Illumina® sequencing, a technique involving the amplification of a hypervariable region (V5-V6 region of the 16S ribosomal RNA) of bacterial DNA and sequencing of the amplified region.
Primary Endpoint Results
Within the first two weeks, yeast fermentate groups usually showed rapid statistically significant improvements for gastrointestinal health when compared to placebo. Analysis of the results within the yeast fermentate group showed those benefits were typically maintained throughout the remainder of the study.
Fewer significant differences were found in the four and six week averages due to a noticeable placebo effect, which seemed to increase over time especially in the severe subgroup. This pronounced placebo effect is not uncommon for gut-related trials with gastrointestinal disorders such as functional constipation.11-14 This may explain why the placebo effect was generally more pronounced in the severe group.
There were more statistically significant improvements within the yeast fermentate group than within the placebo group at weeks four and six, although there were fewer significant differences when compared to placebo. This includes statistically significant improvements for stool consistency and quality of life within the yeast fermentate groups but not within the placebo groups.
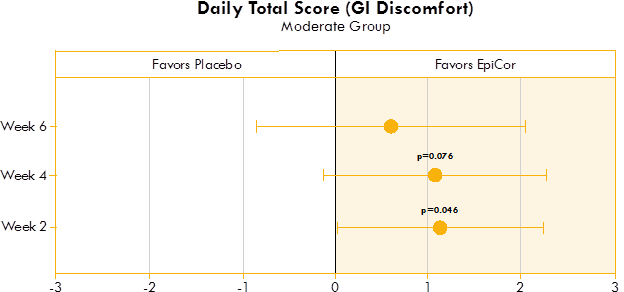
Figure 1. Box plot illustrating the mean difference in GI discomfort in a placebo group and yeast fermentate group.
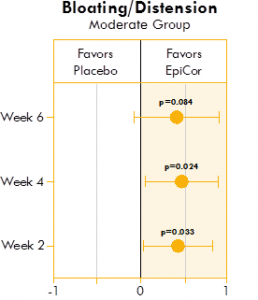
Figure 2. Box plot illustrating the mean difference in bloating and distension in a placebo group and yeast fermentate group.
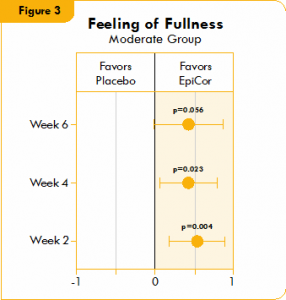
Figure 3. Box plot illustrating the mean difference in feeling of fullness in a placebo group and a yeast fermentate group.
Figures 1, 2, and 3 show the mean differences between yeast fermentate and placebo on gastrointestinal symptoms (as recorded on a daily basis by self-assessment of the severity of GI symptoms on a five-point scale), analyzed with a linear mixed model that takes into account the differences between groups at baseline.
Stool Parameters
As a part of the patient diary, daily stool frequency and consistency were documented using the Bristol Stool Form Scale for a two-week run-in and a six-week intervention phase. Scores were averaged every two weeks.
The Bristol Stool Form Scale comprises seven types of stool: type 1 (separate hard lumps); type 2 (sausage shape lumpy); type 3 (sausage with cracks); type 4 (sausage but soft and smooth); type 5 (soft lobs); type 6 (fluffy and mushy); type 7 (liquid).
Types 1, 2 and 3 are associated with hard and impacted stools which can be linked with dysbacteriosis and chronic constipation. Types 4 and 5 are considered normal or optimal. Types 6 are considered subnormal or suboptimal. Type 7 is associated with diarrhea.
Bristol Stool Form Scale Results
Significant improvements were seen in stool consistency in both the yeast fermentate total cohort group and the severe subgroup compared to placebo. The improvements were seen at week two, showing both a rapid and significant response to yeast fermentate . Also, it should be noted that within all yeast fermentate groups (total, moderate and severe) there were statistically significant improvements in stool consistency over time, while there were no significant improvements over time in any of the three placebo groups (Fig. 4).
Nearly significant improvement (p<0.10) for stool frequency was seen in weeks two and four in the yeast fermentate total cohort group as compared to placebo (Fig. 5). Also, highly significant improvements were seen within all three yeast fermentate groups (total, moderate and severe) over time.
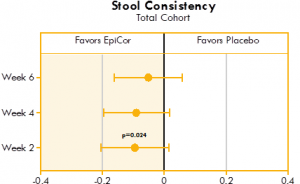
Figure 4. Box plot illustrating the mean differences between yeast fermentate and placebo groups on stool consistency, analyzed with a linear mixed model that takes into account the differences between groups at baseline.
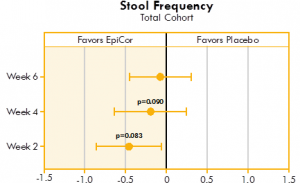
Figure 5. Box plot illustrating the mean differences between yeast fermentate and placebo groups on stool frequency, analyzed with a linear mixed model that takes into account the differences between groups at baseline.
Figures 4 and 5 show the mean differences between yeast fermentate and placebo on gastrointestinal symptoms (as recorded on a daily basis by self-assessment using the Bristol Stool Form Scale), analyzed with a linear mixed model that takes into account the differences between groups at baseline.
Quality of Life Parameters
Quality of life was assessed through a validated Patient Assessment of Constipation Quality of Life (PAC-QOL) questionnaire at baseline and after three and six weeks of intervention.
PAC-QOL Results Showed
- Significant improvement in Physical Discomfort within the yeast fermentate group but not within the placebo group.
- Significant improvement in Psychosocial Discomfort within the yeast fermentate group but not within the placebo group.
- Significant improvement in Satisfaction within the yeast fermentate group but not within the placebo group.
Perceived Stress Parameters
Perceived Stress was assessed through a validated Perceived Stress Scale (PSS) questionnaire at baseline and after three and six weeks of intervention.
PSS Results Showed
- Significant decrease over time for both the total cohort and severe groups within the yeast fermentate group, but not within the placebo group.
- Nearly significant decrease in stress over time between the yeast fermentate and placebo groups (p<0.10) in both the total cohort and moderate subgroup.
Secondary Microbiome Endpoint Results
Total Cohort
Anaerostipes significantly increased in the yeast fermentate groups (total cohort and severe subgroup) when compared to the placebo groups. Anaerostipes is a genus recognized for its health enhancing effects and contains acetate- and lactate-consuming bacteria and butyrate-producing bacteria.15
Moderate Subgroup
Akkermansia muciniphila significantly increased in the yeast fermentate group when compared to the placebo group. Akkermansia muciniphila is important to proper gut function and inversely correlated with metabolic disorders.16
Blautia and Roseburia both significantly decreased in the yeast fermentate group when compared to the placebo group. Blautia and Roseburia are believed to be higher in irritable bowel syndrome (IBS) and constipation-predominant IBS (C-IBS).
Severe Subgroup
Firmicutes significantly decreased while Bacteroidetes relatively increased when compared to placebo, which lead to a decrease in the Firmicutes/Bacteroidetes (F/B) ratio. A two-to-one ratio of Firmicutes to Bacteroidetes has been found in C-IBS patients.17 Prevotella (Family Prevotellaceae) significantly increased when compared to the placebo group. A lower incidence of Prevotella species has been associated to low-fiber diets and insufficient plant-based food consumption, which is a major cause of dysbiosis in the gut of constipated patients.17-19
The significant increase in Prevotella may explain yeast fermentate ’s positive effects on stool frequency and consistency. Bacteroides (Family Bacteroidaceae) significantly increased when compared to the placebo group. The significant increase in Bacteroides may explain yeast fermentate ’s positive effects on stool frequency and consistency.
Conclusion
Building upon published in vitro research showing yeast fermentate has powerful prebiotic effects at only a 500 mg daily dose, a human clinical trial was conducted with 80 healthy adult subjects with symptoms of GI discomfort and reduced bowel movements. Yeast fermentate led to statistically significant improvement of symptoms such as bloating/distension, feeling of fullness and daily total scores in the moderate subgroup.
A significant improvement in stool consistency was observed for the total cohort as well as for the severe subgroup and a nearly significant increase in stool frequency was detected for the total cohort. These effects were accompanied by an improvement in constipation-associated quality of life and general perceived stress, particularly within the moderate subgroup. While improvements were also seen in the severe group, there was a larger placebo effect in this group due to the desire to see an improvement in the more severe symptoms.
The novel findings that yeast fermentate positively modulates the gut microbiota provide additional substantiation for its GI-related efficacy. Yeast fermentate decreased the F/B ratio in the severe subgroup, which is known to be higher in C-IBS. Bacteroidetes, namely members of Prevotellaceae and Bacteroidaceae groups, are associated with acceleration of GI transit increases. The significant increase within the severe group in Bacteroides and Prevotella (both genera in the Prevotellaceae and Bacteroidaceae groups) may explain Yeast fermentate ’s significant effects on stool consistency and nearly significant effects on stool frequency. Bacteroides and Prevotella have also been previously reported to be deficient in constipated patients20. In the moderate subgroup, a significant increase in Akkermansia muciniphila was observed. Reduced levels of this bacterium have been inversely correlated with metabolic disorders as well as with inflammatory conditions like IBS.


















