Bookmark
Bookmark Neuroinflammation and the Connection to Mood and Cognition
Mood disorders and cognitive dysfunction affect hundreds of millions of people worldwide and are major causes of disability.1,2 While some medications and intervention strategies have been developed, many brain disorders are due to a complex combination of factors which can make treatment difficult.2
The role of inflammation, specifically neuroinflammation, has gained more attention in recent years and has been linked to a variety of diseases related to mood and cognition. A better understanding of the role of neuroinflammation in mood and cognition may lead to more effective treatments, including anti-inflammatory approaches, to help reduce symptoms and support brain health.2
Understanding Neuroinflammation
Neuroinflammation is more than inflammation in the brain — it involves a combination of psychological, neuroendocrine, and nervous system changes that affect many components of brain health, including neurotransmitter metabolism, HPA axis function, and microglial cell activation.3 The end result is impaired neuroplasticity and pathological changes to the structure and function of the brain which affects both cognition and mood regulation.3
The specific causes of neuroinflammation can vary from person to person, but they include viral infections, autoimmune disease, mental stress, metabolic dysfunction, inflammation from other organs, environmental toxins, and lifestyle habits which contribute to an inflammatory state.4
Neuroinflammation is thought to be primarily signaled by and propagated by cytokines, chemical messengers in the body that act as immune system signals.3 The abundance of several pro-inflammatory cytokines, including IL-1, IL-6, TNF-α, and IFN-γ, has been associated with psychiatric symptoms, disease severity, HPA axis hyperactivity, and neurotransmitter deficiency.3
Under normal conditions, most cytokines cannot cross the blood brain barrier (BBB) due to their relatively high weight; however, under pathological conditions, peripheral cytokines enter the brain due to increased BBB permeability.3 Cytokines can also bind to peripheral afferent nerve fibers in the vagus nerve which relay the signal to specific brain regions.3
Once they reach the brain, cytokines are transported into microglia and astrocytes, which in turn produce more cytokines. Microglial cells are mediators of inflammatory processes in the brain, producing more pro-inflammatory cytokines and reactive oxygen species (ROS).3 Microglial cells can also recruit monocytes into the brain in response to inflammatory signals which further increases inflammation via their production of pro-inflammatory cytokines.3
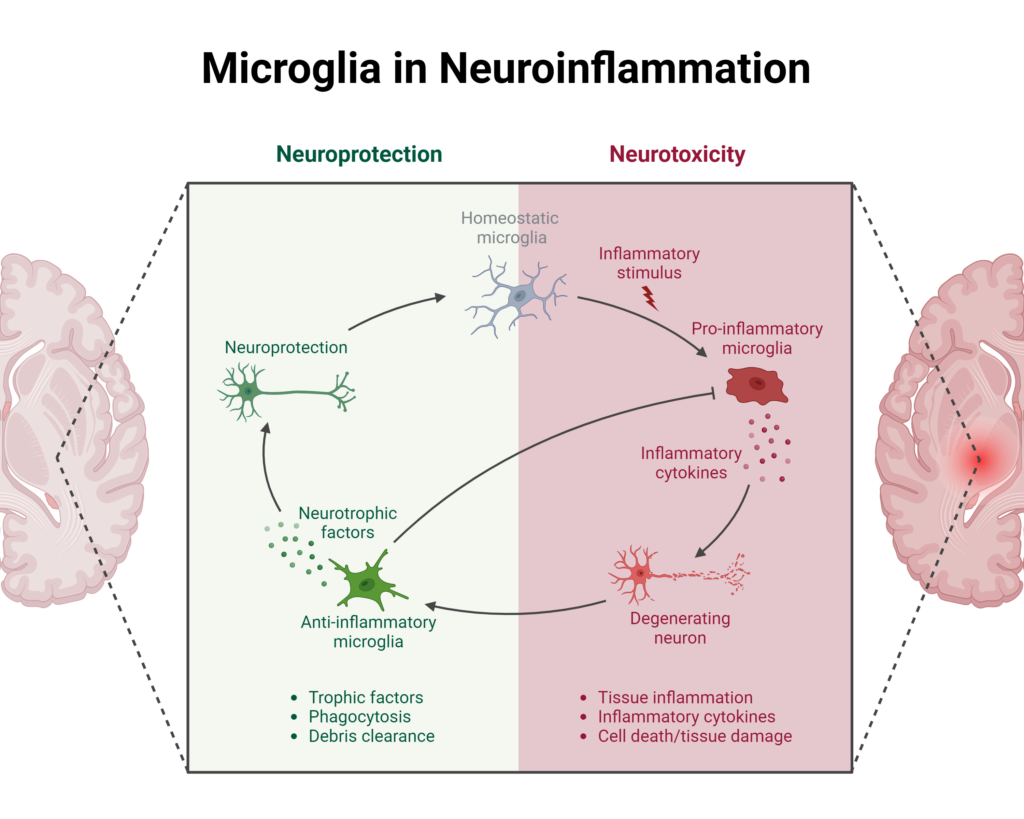
Image created using BioRender.com
The increased concentration of cytokines in the brain has many consequences, including specific effects depending on their location. Cytokines can increase neurotoxic metabolites as well as directly exert neurotoxic effects on brain regions involved in emotional response, neurotransmitter metabolism, neuroendocrine functions, and mood regulation.3
Neuroinflammation can also lead to dysbiosis in the gut which further contributes to brain dysfunction via the gut-brain axis and translocation of bacterial products from the gut.2
Neuroinflammation and Mood Disorders
Several mood disorders have been linked to chronic, low-grade inflammation and some studies have also found higher levels of cytokines specifically during mood episodes in individuals with major depressive disorder and bipolar disorder.2,5 Additionally, over twenty percent of patients with depression and anxiety are affected by neuroinflammation and are more likely to experience a more severe and chronic disorder that is resistant to treatments.6-8
Since identifying neuroinflammation as a key pathway in mood disorders, scientific studies have also revealed that treatment with anti-inflammatory strategies may help improve mood symptoms.2
Mechanistically, pro-inflammatory cytokines that reach the brain are able to alter neurotransmitter metabolism, disturb neural circuits that are involved in mood regulation, and activate the HPA axis.2,3
In the short term, increased HPA axis activity can be beneficial, but chronic activation causes significant changes in glucocorticoid metabolism and anxiety-like behavior.3 In turn, stress can lead to further changes in mood and anxiety, activate the immune system to increase inflammation via damage-associated molecular patterns (DAMPs) and pattern-associated molecular patterns (PAMPs), and contribute to further microglial activation.3,6
Pro-inflammatory cytokines also decrease the bioavailability of several neurotransmitters, including serotonin and dopamine, which are involved in mood regulation and other functions including body movement and digestion.3
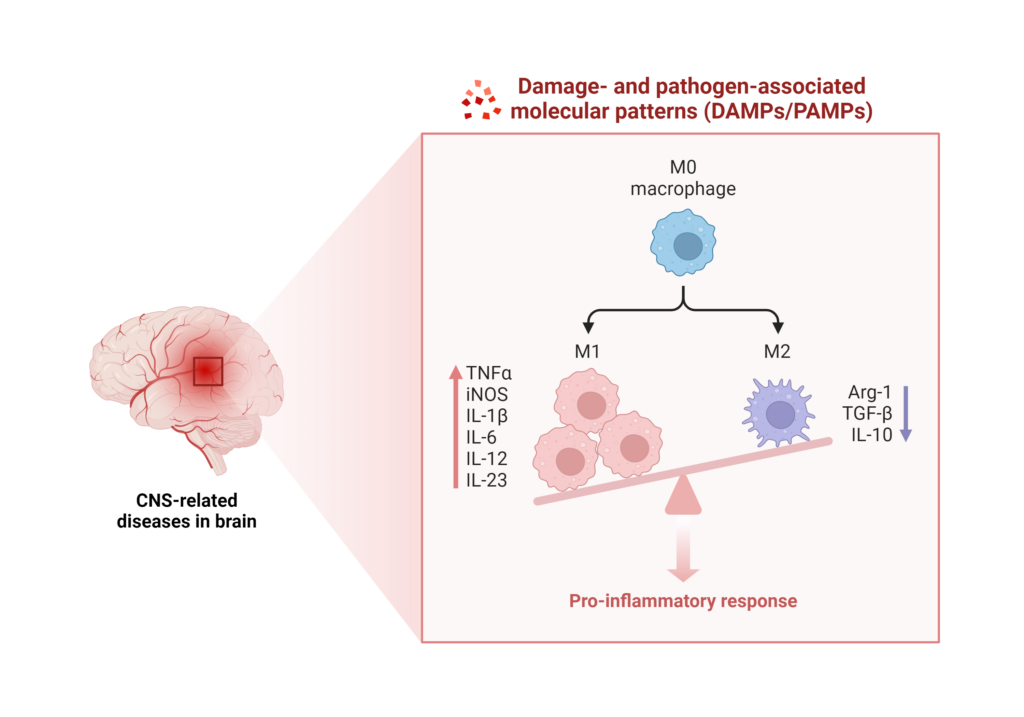
Image created using BioRender.com
Neuroinflammation and Cognitive Function
In addition to impacting mood regulation, neuroinflammation can alter several aspects of neuronal integrity, which ultimately affects cognitive functions including learning and memory.3
In one study, neuroinflammation contributed to both structural and functional disruptions to neuronal networks independent of known mechanisms of Alzheimer’s disease.9 Measures of neuroinflammation, including levels of translocator protein (TPSO), have also been inversely associated with the progression of both mild cognitive impairment and Alzheimer’s’ disease.10
Specific inflammatory cytokines have been linked to changes in cognitive function, anatomy of the brain, as well as to decreased BDNF levels and activity which alters synaptic plasticity in the hippocampus, and ultimately affects memory and learning.11-13
Neuroinflammation also affects microglial cells which are part of the construction and maintenance of healthy neuronal networks, and any damage to these systems can impair cognitive function. Additionally, activated microglial cells modulate neurotrophic factors levels and activate cellular signaling pathways that affect cognitive functions, as well as promote neurodegenerative diseases.13,14
Other mechanisms of neuroinflammation-induced damage include changes in the tryptophan-kynurenine pathway which results in changes in serotonin metabolism as well as decreased neurogenesis and increased neurodegeneration.13
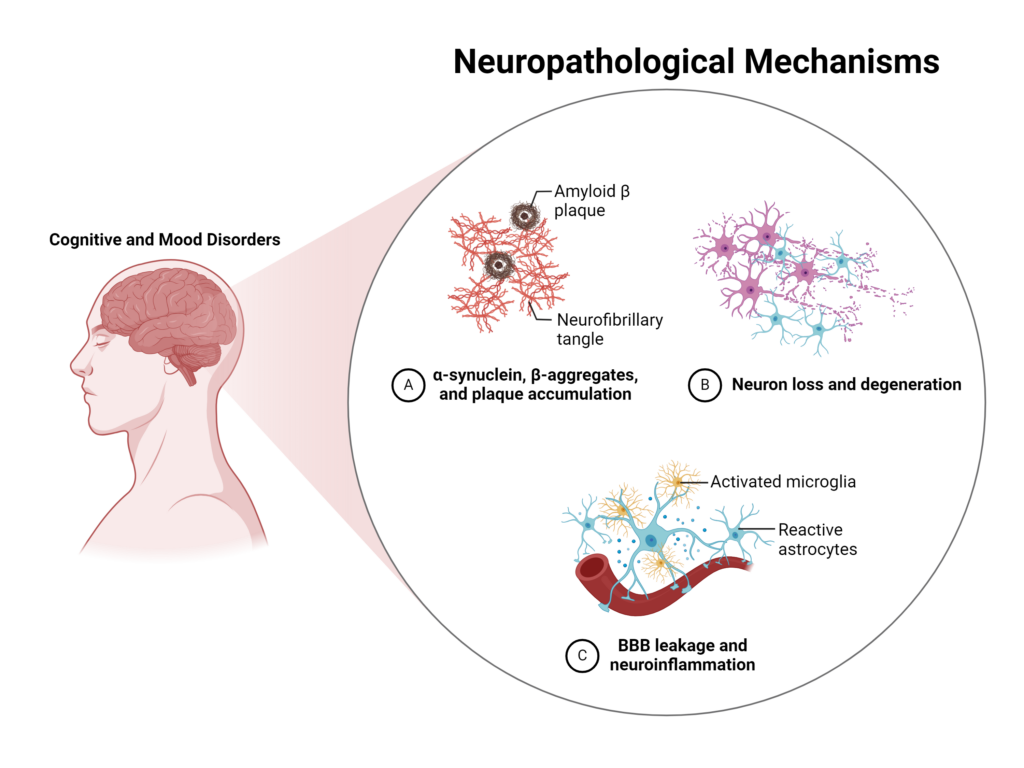
Image created using BioRender.com
Diagnostic Tools and Biomarkers
While higher levels of cytokines in the plasma and cerebrospinal fluid have been linked to neuroinflammation and other options such as the dexamethasone suppression test (DST) can indicate functioning of the HPA axis, clinical evaluation of symptoms is critical to identify patients who may be experiencing neuroinflammation.6
Therapeutic Approaches
For the general population, as well as individuals who may be predisposed to neuroinflammation, adopting lifestyle habits that support healthy inflammation can significantly reduce inflammation throughout the body and support brain health.
Consuming a healthy diet, engaging in regular exercise, and practicing stress management techniques can help reduce inflammation throughout the body. Additionally, dietary supplements with powerful anti-inflammatory and antioxidant actions may also help reduce inflammation, including omega-3 fatty acids.6
Medicinal herbs can also help reduce neuroinflammation and support brain health including curcumin from turmeric (Curcuma longa), Boswellia serrata, and Bupleurum species. These herbs have been found to reduce inflammation, improve serum biomarkers of neuroinflammation, improve cognitive functions, and improve depression by modulating BDNF.15-17
Because of the complex nature of many psychiatric and cognitive illnesses, a multi-tiered approach may help identify patients most at risk of developing neuroinflammatory conditions such as depression and anxiety.
Screening for risk factors and early signs of neuroinflammation may also help intervention begin before symptoms develop or worsen. Teaching patients about lifestyle choices that may reduce neuroinflammation can also help support long-term healthy habits.
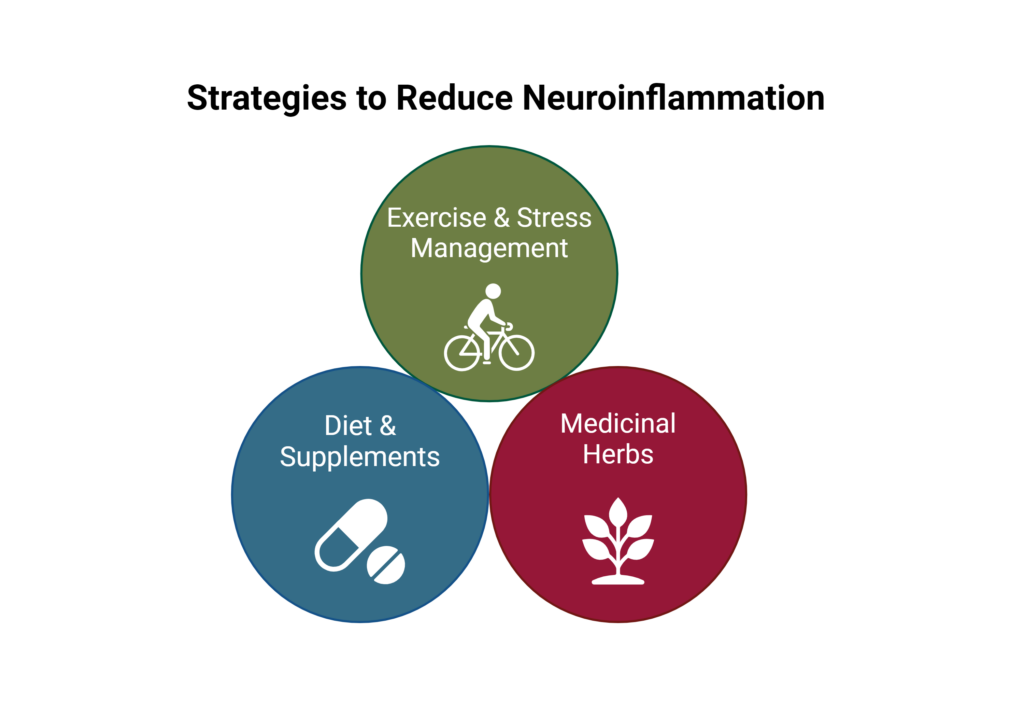
Image created using BioRender.com
While many conditions afflicting the brain have complex pathologies, neuroinflammation can be an important contributor to disease pathogenesis, especially in a subset of individuals with mood and cognitive disorders.
Adoption of anti-inflammatory strategies such as dietary changes, exercise, and anti-inflammatory supplements can support overall health and reduce neuroinflammation.
Did you know Wholistic Matters is powered by Standard Process? Learn more about Standard Process’ whole food-based nutrition philosophy and where to buy.
- Santomauro, D.F., Mantilla Herrera, A.M., Shadid, J., et al. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet, 398:1700.
- Bauer, M.E., Teixeira, A.L. (2021). Neuroinflammation in Mood Disorders: Role of Regulatory Immune Cells. Neuroimmunomodulation, 28(3):99.
- Rhie, S.J., Jung, E.-Y., Shim, I. (2020). The role of neuroinflammation on pathogenesis of affective disorders. J Exerc Rehabil, 16(1):2.
- Sun, Y., Koyama, Y., Shimada, S. (2022). Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front Aging Neurosci, 14:903455.
- Sayana, P., Colpo, G.D., Simões, L.R., et al. (2017). A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res, 92:160.
- Hassamal, S. (2023). Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psychiatry, 13:1130989.
- Guo, B., Zhang, M., Hao, W., Wang, Y., Zhang, T., Liu, C. (2023). Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Translational Psychiatry, 13:5.
- Haroon, E., Daguanno, A.W., Woolwine, B.J., Goldsmith, D.R., Baer, W.M., Wommack, E.C., et al. (2018). Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology, 95:43.
- Leng, F., Hinz, R., Gentleman, S., Hampshire, A., Dani, M., Brooks, D.J., Edison, P. (2022). Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Molecular Psychiatry, 28:1303.
- Bradburn, S., Murgatroyd, C., Ray, N. (2019). Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res Rev, 50:1.
- Barbosa, I.G., Ferreira, R.A., Rocha, N.P., et al. (2018). Predictors of cognitive performance in bipolar disorder: the role of educational degree and inflammatory markers. J Psychiatr Res, 106:31.
- Mohite, S., Cordeiro, T., Tannous, J., et al. (2020). Eotaxin-1/CCL11 correlates with left superior temporal gyrus in bipolar disorder: a preliminary report suggesting accelerated brain aging. J Affect Disord, 2020;273:592–6.
- Fourrier, C., Singhal, G., Baune, B.T. (2019). Neuroinflammation and cognition across psychiatric conditions. CNS Spectr, 24(1):4.
- Ahmad, M.A., Kareem, O., Khushtar, M., Akbar, M., Haque, M.R., et al. (2022). Neuroinflammation: A Potential Risk for Dementia. Int J Mol Sci, 23(2):616.
- Das, S.S., Gopal, P.M., Thomas, J.V., Mohan, M.C., Thomas, S.C., Maliakel, B.P., et al. (2023). Influence of CurQfen®-curcumin on cognitive impairment: a randomized, double-blinded, placebo-controlled, 3-arm, 3-sequence comparative study. Front Dement, 2:1222708.
- Karima, S., Aghamollaii, V., Baram, S.M., Balenci, L., Lanctot, K.L., Kiss, A., et al. (2023). Boswellic Acids Improve Clinical Cognitive Scores and Reduce Systemic Inflammation in Patients with Mild to Moderate Alzheimer’s Disease. J Alzheimers Dis, 94(1):359.
- Wang, X., Feng, Q., Xiao, Y., Li, P. (2015). Radix Bupleuri ameliorates depression by increasing nerve growth factor and brain-derived neurotrophic factor. Int J Clin Exp Med, 8(6):9205.