What is Inflammation?
Inflammation is foremost a defense mechanism against infection and tissue damage. However, if left unresolved it can progress to chronic inflammation and contribute to the development of diseases. Instead of trying to block or rid the body of inflammation, the goal should be to support healthy inflammation and resolution.
Cellular Signaling in Inflammation
Inflammation involves a cascade of molecular reactions and cellular activity to repair and restore damaged tissue, from a small cut to childbirth.1 In order to repair the tissue and resolve inflammation, cells, proteins, and signaling molecules must coordinate within cells as well as with the extracellular matrix and vasculature.2 Restoring normal vasculature ensures that tissue oxygenation is sufficient, because without it, atrophy or fibrosis can occur.2 If the extent of fibrosis is significant, it may interfere with organ function.2
Atrophy: the loss of organ tissue such as liver or muscle
Fibrosis: excess connective tissue development in organs
Mediators and mechanisms of inflammation
When a foreign pathogen invades or tissue damage occurs, the innate immune system is activated by compounds called pathogen-associated molecular pattern (PAMPs) or damage-associated molecular pattern (DAMPs) molecules.3 The body responds by enhancing blood flow to the inflamed area, enhancing vascular permeability, and initiating phagocytic leukocyte migration to the surrounding tissue.1 Newly generated vessels and larger arterioles enhance the blood flow to help deliver compounds, including leukocytes and plasma mediators, to the site.1 Immune cells, including neutrophils, macrophages, and mast cells, can then find the pathogens and eliminate them, assisted by compounds in the plasma including antibodies.1
The generalized inflammatory response involves chemical and inflammatory mediators, an acute phase response, recruitment of immune cells, and finally the end to the stimulus and healing.1 Specifically, this involves the recruitment of granulocytes to get rid of the pathogens, production of pro-inflammatory mediators, specifically the pro-inflammatory cytokines TNFα, IL-1β, and IL-6, and lipid mediators including prostaglandins and leukotrienes.3,4 Other critical inflammatory mediators include NF-κB and toll-like receptor 4 (TLR4), important regulators in the inflammatory cascade. The coordinated responses serve to clear the pathogen and repair damaged tissue via an acute inflammatory response that can last from hours to days.3
Immune cells are critical to healthy inflammation.5 Upon recognition of a stimuli, including excess nutrients, immune cells will secrete cytokines and chemokines to the affected area.6 If the condition persists, antigen-presenting cells and B- and T-lymphocytes (B and T cells) will be released to help fight off the stimulus.6 Other immune cells that are critical to inflammation include macrophages. In the beginning of an inflammatory response, macrophages tend to be pro-inflammatory, whereas they transition to anti-inflammatory properties later in the process.2 T cells are also an important part of the immune system because they secrete cytokines and control immune reactions. Because T cells contribute to inflammation but T cell deficiency can prolong inflammation, a careful balance of T cells is required for healthy inflammation.2
The cyclooxygenases (COX) are critical enzymes in inflammatory pathways and are common targets of non-steroidal anti-inflammatory drugs.3 COX1 and COX2 are involved in the conversion of unsaturated fatty acids, including arachidonic acid, into prostaglandins, which mediate both acute and chronic inflammation and influence cytokine actions.3 PAMPs, DAMPs, and pro-inflammatory cytokines can also induce the expression of COX genes.3 Increased expression of COX2 is commonly found at the site of inflammation but also in tissues with chronic inflammation including the joints of rheumatoid patients and in tumors of cancer patients.3
Oxidative stress is another piece of the inflammation puzzle. Reactive oxygen species (ROS) are natural byproducts of hundreds of metabolic reactions that take place in the body every day. ROS are not inherently bad or dangerous, but when they accumulate and the oxidative balance switches to oxidative stress, it can trigger an inflammatory response.7 ROS can also regulate the production of inflammatory molecules including cytokines, chemokines, and pro-inflammatory transcription factors.7
The consumption of nutrients in excess also contributes to inflammation, including through oxidative stress pathways.8 The consumption of an unhealthy, high calorie diet will cause an increase in calories and nutrients being stored in adipose tissue, and it is often accompanied by a simultaneous decrease in energy expenditure.9 This can remodel adipose tissue, resulting in dysfunction which may result in dysregulated energy metabolism, altered secretion of hunger hormones, and an influx of inflammatory cytokines.9 The increase in fat tissue also results in increased oxidative stress, further contributing to inflammation.8,9
HOW CAN YOUR DIET IMPACT HOW YOUR BODY REGULATES INFLAMMATION?
Inflammation has a bad reputation but it can actually be a healthy response to challenges your body faces, such as infection, illness or injury. Acute inflammation, for example, is a normal, protective response to injury.
However, inflammation can become your enemy if it’s causing your body to overreact to stressors, creating a chronic, continuing natural inflammation response. Your lifestyle habits, including diet, nutrition, and exercise, can all affect the way your body addresses these challenges.

Phases of Inflammation
The inflammatory process, when working properly, consists of two phases: initiation and resolution. The initiation of inflammation occurs in response to an activator, which can vary from infectious agents to radiation to diet.7 For example, dietary N-glycolylneuraminic acid (Neu5Gc) comes from red meat and dairy products.2 Due to a genetic mutation, some humans recognize Neu5Gc as a foreign body which then triggers an inflammatory response upon consumption. The resulting inflammation promotes angiogenesis and oncogenesis via COX2 pathways.2 Other triggers such as alcohol, stress, cleaning products, or tobacco, can activate inflammation directly or indirectly through influencing the production of inflammatory molecules.7 In response, mediators begin to respond to the site of damage to help clear pathogens and debris and restore homeostasis.
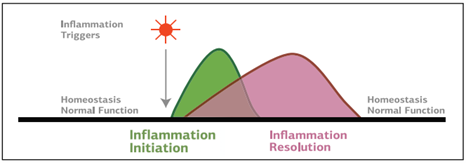
Under optimal conditions, the next phase of inflammation is resolution. Resolution of the acute inflammatory response involves decreased leukocyte recruitment, stimulation of efferocytosis or the removal of cellular debris, clearance of PAMPs and DAMPs, repaired tissue damage, and replenishment of blood and lymphatic vessels.3,10 For bacterial infections, resolution also includes pathogen clearance.10 The cytokines IL-10 and TGF-β are two of the most important mediators of resolution.2 They influence cellular components including immune complexes, prostaglandins, and apoptotic cells.2
The resolution phase of inflammation was previously thought to be a passive process where molecules dissolved or were removed which led to a lack of an inflammatory response. However, it has now been demonstrated that resolution is an active process.2,10 During the later phase of the inflammatory process, molecules that signal via specific receptors or block inflammatory components are both synthesized and released, leading to the resolution of inflammation.2 There are at least 81 genes that contribute to resolving inflammation including those that regulate apoptosis and the clearance of apoptotic cells, cytokines and cell surface receptors, and membrane and intracellular proteins of immune cells.2The resolution of inflammation is also affected by secreted factors including regulatory T cells of the immune system, cytokines, and bioactive lipid mediators.2,10 As such, if expression is reduced or their release is delayed, it may contribute to non-resolving inflammation.2
Pro-resolving mediators are crucial to resolution
Specialized pro-resolving mediators (SPMs) are fatty acid-derived bioactive lipids that contribute to the resolution of inflammation.10 They can be derived from eicosapentaenoic acid (EPA), docososahexaenoic acid (DHA), and docosapentaenoic acid (DPA) and include molecules such as resolvins, maresins, and protectins.10,11 When inflammation begins, many of these bioactive lipids are synthesized by cells including neutrophils and macrophages and mediate the inflammatory response.10
SPMs are commonly considered pro-inflammatory during the earlier stages of inflammation but switch to promoting anti-inflammatory effects later in the response.2 For example, the lipoxygenase enzymes, critical synthesizers of lipid mediators, can act as pro-inflammatory or anti-inflammatory depending on the location and timing.10 For example, in a process called lipoxygenation, the omega-6 fatty acid arachidonic acid can be converted into leukotrienes via 5-lipoxygenase and contribute to pro-inflammatory signals.11 However, double lipoxygenation of arachidonic acid results in the formation of a class of compounds call lipoxins, which help protect against non-resolving inflammation and tissue damage.10, 11
Acute and Chronic Inflammation
Acute inflammation is a productive reaction in the body because it will attempt to destroy and remove potentially dangerous pathogens such as bacteria or viruses. If there is a defect in the resolution of inflammation, it can persist and chronic tissue damage begins, leading to dysfunction and death in organs.1,10 Chronic inflammation can last anywhere from weeks to years.3 Acute and chronic inflammation are not mutually exclusive events- both can coexist over long periods of time, in conditions such as ulcerative colitis.2
Inflammation can be sustained and become chronic through several mechanisms including positive feedback mechanisms that allow for self-amplification of inflammatory responses, suppression of negative feedback mechanisms that would prevent resolution, and further recruitment, activation, transformation, and synergistic interaction of cells involved in inflammation which sustain pro-inflammatory cytokine signaling at the site of inflammation.3 Characteristics of acute versus Chronic inflammationinflammation are shown in the table below:1
| Acute Inflammation | Chronic Inflammation |
| Shorter in duration | Longer time course |
| Immune response is nonspecific | Specific immune response |
| Stereotyped response | Variable response |
| Fluid production as response | Fibrous tissue produced |
| Occasionally necrosis | Always necrosis |
The Endocannabinoid System and Inflammation
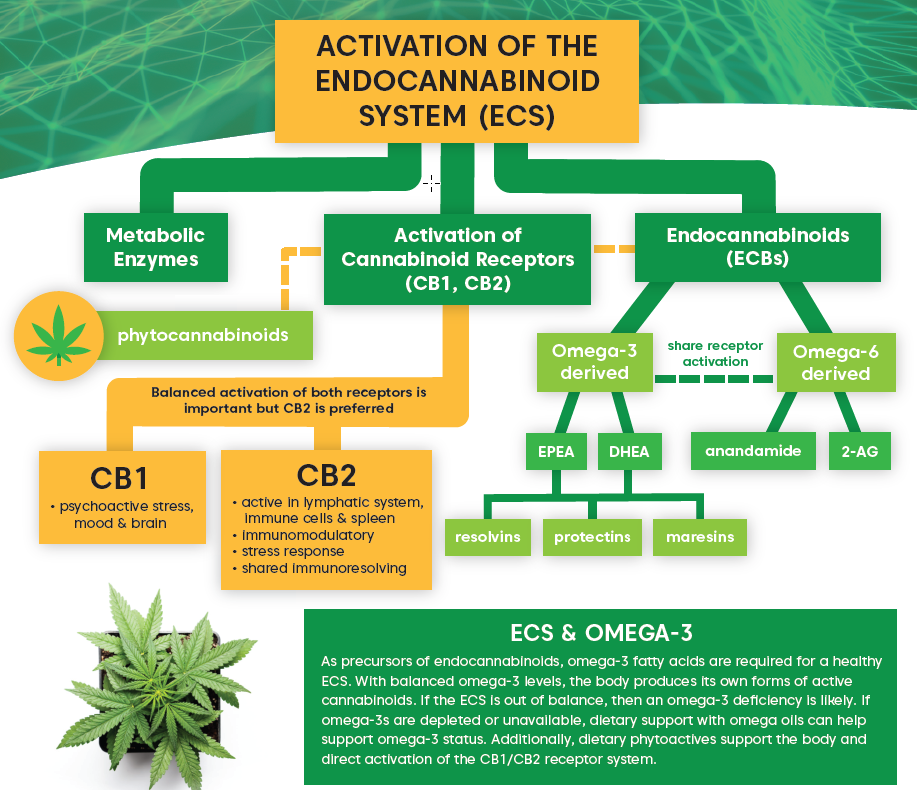
The endocannabinoid system is an important player in inflammation. In immune cells, activation of the cannabinoid receptor CB2 mediates their activation and the secretion of pro-inflammatory mediators.12 CB2 is also able to suppress pro-inflammatory cytokines and harmful secretions as well as regulate immune cell adhesion and migration, helping to resolve localized inflammation.12
Studies have demonstrated that cannabinoids, including phytocannabinoids, possess anti-inflammatory effects.12 The endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) assist in resolving inflammation by reducing pro-inflammatory cytokines and relieving acute inflammation.12 Endocannabinoids can also regulate both innate and adaptive immune activity.12 Further, immune cells can synthesize and release endocannabinoids which continues the cycle and affects tissue inflammation.12 Factors such as a poor diet and lack of exercise can affect the body’s ability to produce AEA and 2-AG, which could negatively impact the balanced interplay of inflammation and resolution.
HOW IS THE ENDOCANNABINOID SYSTEM (ECS) INVOLVED IN THE REGULATION OF INFLAMMATION?
What are the major components of the endocannabinoid system (ECS)? How do omega-3 fatty acids and the ECS act synergistically? What are they key functions of the ECS in the human body? This helpful infographic provides a quick “ECS 101,” useful for a variety of educational situations. Download and print out your free copy today.
Supporting Healthy Inflammation and Resolution
Non-resolving inflammation involves many complex mediators, and there is not always an identifiable stimulus that sustains the inflammatory response. Inflammation can fail to resolve for several reasons including inadequate production of resolution mediators or a prolonged and excessive response.2 The most direct cause of non-resolving inflammation is a persistent stimulus, such as bacteria or asbestos particles that promote chronic inflammation.2 Many forms of chronic inflammation are also heterogenous and depend on the organ where it is occurring.2
There are several anti-inflammatory medications for pain or inflammatory conditions. However, instead of opposing inflammation, which is a normal physiological process, a better option is to support inflammation. Because inflammation functions to protect the body from injury, invasion, and infection, broadly opposing inflammation with anti-inflammatory medications may not have the intended effect. The goal should be to support the appropriately timed initiation and resolution of inflammation by supporting rather than suppressing inflammatory pathways.
The Role of Inflammation in Diseases
Several diseases can arise from or progress due to non-resolving inflammation, including:1-7,10
- Atherosclerosis
- Chronic obstructive pulmonary disease
- Obesity
- Cancer
- Multiple sclerosis
- Asthma
- Inflammatory bowel disease
- Rheumatoid arthritis
- Arterial diseases including hypertension
- Alzheimer’s disease
- Osteoarthritis
- Depression
Mechanisms of action
There are several molecular links between inflammatory processes and diseases, including inflammatory molecules and transcription factors such as chemokines, C-reactive protein, and cytokines.7 NF-κB and TLR4 are also two key connections between inflammation and disease states.7 For example, TLR4 can recognize free fatty acids, which are released from adipose tissue.13 Upon TLR4 activation, more cytokines will be released, creating a feedback loop where the adipose tissue becomes a site of prolonged production of inflammatory cytokines.13
Another link between inflammation and disease states is through cytokines and oxidative stress. Under chronic inflammatory conditions, cytokines and reactive oxygen species are released in excess.13 When free radicals are produced in excessive, they can initiate a chain reaction that leads to damage to the living cell.1 The inflammatory cells that are present can further contribute to inflammation through the release of inflammatory mediators including cytokines, arachidonic acid, and chemokines.1 This inflammatory-oxidative stress cycle can harm nearby cells and cellular components including DNA, proteins, and lipids, leading to cell death and damage to organs.1,13
Inflammation in cancer
Chronic inflammation can act as a precursor to tumor formation- gastritis, bronchitis, and colitis are precursors to gastric, lung, and colon cancer, respectively.7 One of two primary pro-inflammatory transcription factors, NF-κB, is critical to the inflammatory process and regulates over 500 cancer-related genes.7 Chronic inflammation also contributes to other aspects of tumor development including proliferation, invasion, metastasis, and angiogenesis.7 Additionally, some types of cancer can thrive on the angiogenic compounds released by inflammatory cells. In response, tumor cells release chemokines which further activate proteins to produce persistent inflammatory stimulation.2
Inflammation in atherosclerosis
Atherosclerosis is characterized by low-grade, chronic inflammation in the walls of the arteries as well as an imbalance between pro-resolving mediators and pro-inflammatory mediators.10 As chronic inflammation persists, lipids and inflammatory cells accumulate along with other immune cells, forming an atherosclerotic lesion.10 This leads to DAMP-mediated inflammation and tissue injury.10 The subsequent infiltration and accumulation of lipoproteins and leukocytes is the driving force of atherosclerotic lesion growth.10 As such, reducing plasma lipoproteins can help reduce the formation of atherosclerotic plaques but it is also important to address underlying inflammation.10
Obesity and inflammation
Low-grade, chronic inflammation may cause the progression of obesity however, obesity leads to prolonged, non-resolving inflammation, creating a vicious cycle.6 Pro-inflammatory markers have been found to be significantly higher in obese patients compared to healthy individuals and the mechanisms are likely to be complicated and diverse.6 One of the major mechanisms underlying inflammation in obesity is the increased production of ROS in adipocytes, vascular smooth muscle cells, and endothelial cells in response to excessive caloric intake.2 Excess ROS disrupt the mitochondrial electron chain, causing further increased production of ROS as well as pro-inflammatory molecules. This leads to the accumulation of macrophages and adipocyte necrosis followed by an increase in macrophages and inflammatory cytokines.2 However, these pro-inflammatory molecules do not stay sequestered to the adipose tissue- they circulate and affect other tissues including vasculature health in blood vessels and appetite regulation in the brain.2
Inflammation and aging
The role of inflammation in aging processes and aging-related diseases, called “inflamm-aging,” is heavily influenced by the presence and accumulation of senescent cells. Senescence involves the arrest of cellular replication and telomere shortening, and senescent cells display a senescence-associated secretory phenotype (SASP) which includes increased expression and release of pro-inflammatory cytokines, chemokines, and proteases.14 When the immune system cannot rid the body of senescent cells, they accumulate in organs and lead to a sustained pro-inflammatory state and contribute to diseases including atherosclerosis and cancer.14 Immunosenescence is also part of the aging process, where immune cells of both the innate and adaptive immune system undergo senescence. This affects the many functions of the immune system including defense against pathogens as well as long-term immunity – and also provides one explanation for why elderly individuals experience a higher risk of infections.14
The Standard American Diet Promotes Inflammation
The Standard American Diet (SAD), characterized by high consumption of red meat, processed foods, salt, and sugar and low intake of fruits, vegetables, and whole grains, contributes to an inflammatory state. This results in high levels of saturated fats and omega-6 fatty acids being consumed while omega-3 fatty acids in the diet stay relatively low. The major mechanisms through with the SAD influences inflammation is through the low abundance of omega-3 fatty acids, modulating gut microbiota, and nutrients directly influencing inflammatory pathways.15
Omega-3 fatty acids and inflammation
The SAD is notably low in omega-3 fatty acids and abundant in omega-6 fatty acids which is especially detrimental to supporting healthy inflammation as omega-3 fatty acids tend to suppress inflammation while omega-6 fatty acids contribute to pro-inflammatory pathways.7,16 In the Standard American Diet, the ratio of omega-6 to omega-3 is close to 20:1, which promotes inflammation and hinders the formation of pro-resolving mediators.16
Dysbiosis from high fat intake
Chronic consumption of the SAD results in negative changes in the gut microbiota, selectively increasing the abundance of harmful bacteria while reducing the numbers of beneficial bacteria that help support the health of the body.16 This imbalance of “good” and “bad” bacteria is referred to as dysbiosis. Dysbiosis results in gut barrier dysfunction, increased intestinal permeability, and the leakage of toxic metabolites into circulation which contributes to low-grade systemic inflammation.16 High intake of fructose, found in added sugars, can also result in bacterial overgrowth in the intestines, leading to elevated endotoxins which active TLR4.13
Additionally, consumption of SAD reduces the overall concentrations of short chain fatty acids (SCFAs).16 SCFAs are produced by the bacteria feeding on dietary components that are not digested by human intestinal cells. They have many useful functions including providing energy to intestinal cells and reducing local inflammation and intestinal permeability.16 The SAD reduces overall SCFA content but the dysbiosis that occurs due to SAD consumption also leads to a decrease in beneficial SCFAs.16
Crosstalk between microbiota and inflammatory processes
The effects of dysbiosis do not stay within the gut. There is crosstalk with different organs that allow the microbiota to affect inflammatory processes throughout the body. Alterations in gut microbiota from high fat diet feeding can change the expression of both inflammatory and metabolic genes including inducing expression of COX2.16 Dysbiosis can also induce inflammation via the NF-κB pathway, increase expression of the genes that produce pro-inflammatory cytokines, and increase the activation of immune cells including neutrophils and macrophages.16
Fat-driven inflammatory processes
Beyond influencing the gut microbiota, the Standard American Diet can exert inflammatory effects due to its composition and the ability of the immune system to respond to an excessive supply of nutrients. Continuous over-eating with no extended fasting time leads to increased production of ROS and oxidative stress.16 Dietary fatty acids can also:16
- significantly alter the balance of the endocannabinoid system
- stimulate both local and systemic inflammation
- increase expression and concentration of pro-inflammatory cytokines
SAD consumption also leads to a decrease in gut peptides which regulate appetite and gut motility, help maintain gut barrier integrity, and possess anti-inflammatory properties.16 Dietary fatty acids can directly influence intestinal barrier integrity and activate TLR4, causing the release of pro-inflammatory cytokines.16 Finally, the excess adipose tissue that may form due to consumption of SAD can cause immune cells in the adipose tissue to stimulate and sustain inflammation.16
Herbs and Nutrients Associated with Inflammation
Anti-inflammatories in Western medicine
Many therapeutics seek to combat chronic diseases that involve inflammation but they often only focus on a specific target, limiting their efficacy.7 These drugs tend to be expensive and may have side effects when taken for a long time.5,7 Interestingly, more than 70 percent of the drugs that have been introduced over the years for chronic inflammation-related conditions were derived from natural sources including cereals, fruits, nuts, spices, and vegetables.7 A different approach is to focus on healthy resolution of inflammation through dietary intervention.
Spices and herbs
Many herbs and botanicals modulate inflammation. Curcumin is one of the most well-known and well-studied compounds for suppressing inflammation and reducing oxidative stress.7 Curcumin is a component of the herb turmeric and can affect inflammation both directly and indirectly, including by directly binding to and inhibiting the actions of COX1, COX2, and TNF-α.7
Boswellia serrata also possesses inflammation-modulating properties, including inhibiting the synthesis of the pro-inflammatory enzyme 5-lipoxygenase.17 It may also reduce cytokine levels and inhibit inflammatory-related proteins including NF-κB.17 Human trials have demonstrated positive effects of taking B. serrata supplements on various inflammatory conditions including osteoarthritis.17 Other medicinal plants that possess potent anti-inflammatory effects include Siberian ginseng, hardy kiwi vine, Crataeva nurvala, and Schisandra chinensis.1
Dietary nutrients
Omega-3 fatty acids are vital to healthy inflammation due to their role in the formation of pro-resolving mediators.11 They also contribute to the resolution of inflammation through other mechanisms including modulating the immune response, reducing oxidative stress, and affecting cellular signaling through altering gene expression.11,15 In humans, consumption of the essential omega-3 fatty acids DHA and EPA decreased circulating markers of inflammation and resulted in changes in expression of over 1,000 genes including decreased expression of genes involved in inflammatory pathways.15 Higher intake of omega-3 fatty acids has also been associated with a decreased risk of developing Alzheimer’s disease and treatment with EPA and DHA led to reduced release of inflammatory factors.15
Consumption of a diet that is high in fruits and vegetables can help prevent chronic inflammation and resolve acute inflammation due to its high content of anthocyanins and flavonoids.18 A diet high in anthocyanins may help reduce inflammation as anthocyanins possess antimicrobial, antioxidative, and anti-inflammatory properties.6 Anthocyanins are able to eliminate ROS and activate antioxidant enzymes as well as inhibit NF-κB, which helps to restore oxidative balance.6 Supplements containing anthocyanin mixtures reduced inflammatory markers in patients with high cholesterol and dietary anthocyanins from black soybean and red orange juice decreased inflammatory markers as well as improved metabolic markers.6
Quercetin is a flavonoid that has been shown to be anti-inflammatory, anti-carcinogenic, and anti-viral, as well as work as an antioxidant.18 Quercetin is found in apples, berries, brassica vegetables, onions, grapes, tea, and tomatoes; however, bioavailability is very low.18 Mechanistically, quercetin inhibits TNF-α production in macrophages, as well as inhibits production of COX and LOX enzymes, which produce pro-inflammatory compounds.18 It can also suppress leukocyte recruitment, decrease chemokine levels, and increase the activity of antioxidant enzymes.18
Other dietary components with healthy inflammation-modulating effects include capsaicin, curcumin, epigallocatechin gallate (EGCG), and resveratrol through inhibiting NF-κB activation and reducing inflammatory cascades.7 EGCG is found in green tea and may also help protect against several chronic diseases.7 Other dietary components including garlic, ginger, onions, bergamot orange, broccoli, and cinnamon also exert anti-inflammatory effects and improve other metabolic markers.19
Plant-based diets tend to be higher in antioxidants which can help reduce oxidative stress and prevent the development of diseases such as cancer and cardiovascular disease.1 Trace minerals including copper, zinc, magnesium, manganese, and selenium also function as part of antioxidant systems, as well as antioxidants from vitamins including tocopherols, carotenoids, and ascorbic acid.1 Together, these contribute to antioxidant systems in the body to keep inflammation in balance.1 Many natural products that contain antioxidants also contain flavonoids and phenolic compounds that support healthy inflammation and resolution by reducing the production of pro-inflammatory mediators.1
Inflammation is a complex mechanism in the body to destroy invading pathogens and repair damaged tissue. When inflammatory pathways and mediators are functioning optimally, they will respond to a trigger and resolve in an appropriate amount of time. However, if inflammation does not resolve, it can lead to chronic inflammation and related conditions that can affect health. Instead of relying solely on therapeutics that block inflammation, support healthy inflammation through a plant-based diet, including herbs and spices that help promote resolution.
WHAT ROLE DO MEDICINAL HERBS PLAY IN HOW THE BODY REGULATES INFLAMMATION?
Herbs can play a useful role in the management of inflammation and pain that often accompanies inflammation. However, the selection of anti-inflammatory herbs needs to be appropriate to the condition under treatment. Individuals are advised to ask their physician about what herbs.








