Metabolic Detox 101
Metabolic Toxin Exposure and Removal
The human body is exposed to endogenous metabolic toxins – both environmental toxicants and toxins – on a daily basis. The environment contains approximately 80,000 novel chemicals, all registered with the United States Environmental Protection Agency (EPA) since World War II. However, many chemicals have not been thoroughly vetted for risk to human health.1
Toxin exposure can put pressure on the body’s natural metabolic detoxification capacity. Improper clearance of toxins may play a role in obesity, cardiovascular disease, neurocognitive concerns, immune dysfunction, chemical intolerance, and reproductive and developmental concerns.1-5 The human body has a well-defined, three-phase detoxification system to eliminate toxins.
Toxins exert many detrimental effects on the body in both the short- and long-term and affecting body systems, specific organs, and even down to individual cells. Certain toxins are able to poison enzyme systems, prevent the production of the oxygen-carrier hemoglobin, weaken protection against oxidative stress, damage vital organs including the liver, and contribute to inflammation.6,7 The degree of exposure is an important determinant in the specific health effects that may arise.7
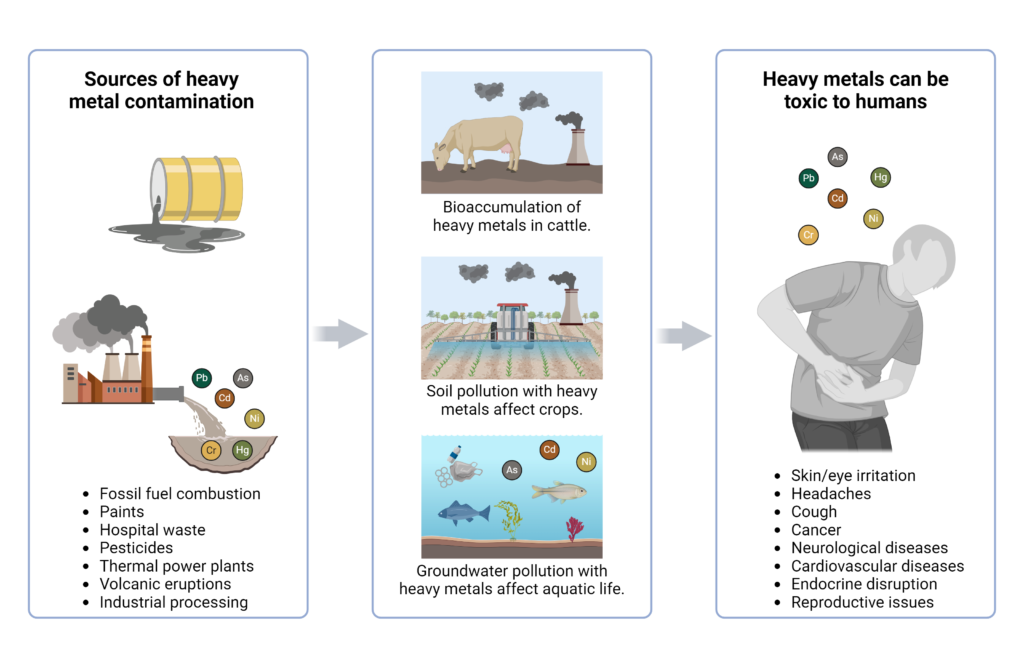
The specific type of toxins can result in differential effects on the body. For example, heavy metals, such as cadmium, mercury, lead, and chromium, are particularly toxic to the kidneys due to their damaging effects on renal tubules. Exposure to heavy metals can come through consumption of contaminated fish, exposure to preservatives, or even dental amalgam fillings.7
Other dangerous toxins include those from the environment, such as pesticides, microplastics, industrial chemicals, and bacterial endotoxins can also have negative effects on the body. Many of these biological pollutants act as endocrine disrupters and can result in headaches, coughing, chest pain, and skin irritation.7
Long-term toxin exposure contributes to the development and progression of serious diseases including cardiovascular disease, Parkinson’s disease, and cancer. For cardiovascular and neurological diseases, ultrafine particles enter the body and reach the circulatory system or brain, respectively, where they can cause oxidative stress, inflammation, and dysfunction.7
Over time, this results in significant damage to the cardiovascular and central nervous systems, including disrupting the blood brain barrier, inducing mitochondrial dysfunction, and damaging DNA.7 Interestingly, environmental causes of cardiovascular disease account for more cases than those due to metabolic issues, tobacco use, or behavioral risk factors, while environmental toxins have been linked to between 70 and 80 percent of all cancer forms.7 Carcinogenic and mutagenic toxins damage DNA and alter methylation of genes, leading to activated cancer signaling pathways while silencing tumor suppressor genes, leading to cancer development.7
Toxins Vs. Toxicants
The word “toxin” is defined as a harmful compound made by bacteria, plants, or animals, while the word “toxicant” refers to harmful compounds made by humans or compounds introduced into the environment by human activities. Each toxic substance has a defined dose or concentration at which it produces a harmful effect.
Toxicants are harmful exogenous compounds that enter the body via air, drinking water, and food. Toxins, on the other hand, could be either exogenous (found in the environment) or endogenous (produced by the body). In this discussion, the word “toxin” will be used to indicate either toxins (biological source) or toxicants (chemical source).
Metabolic Detoxification
Key biochemical processes responsible for the clearance of toxins from the body make up the biotransformation process, also called the metabolic detoxification system. This system is comprised of three different pathways.
The detoxification system is highly dependent on proper nutrient support for optimal functioning. Nutritional support for the biotransformation system is extremely important for any detoxification program. Detoxification is an energy-dependent process and maintenance of adequate energy supply is crucial.
Fasting or poor nutritional support during a detoxification program may have many adverse health effects, including:
- Decreased energy production
- Brain fog
- Mood and sleep difficulties
- Breakdown of lean tissue
- Up-regulation of detox Phase I enzyme activities with a concomitant increase in oxidative stress
- Decreased levels of Phase II co-factors
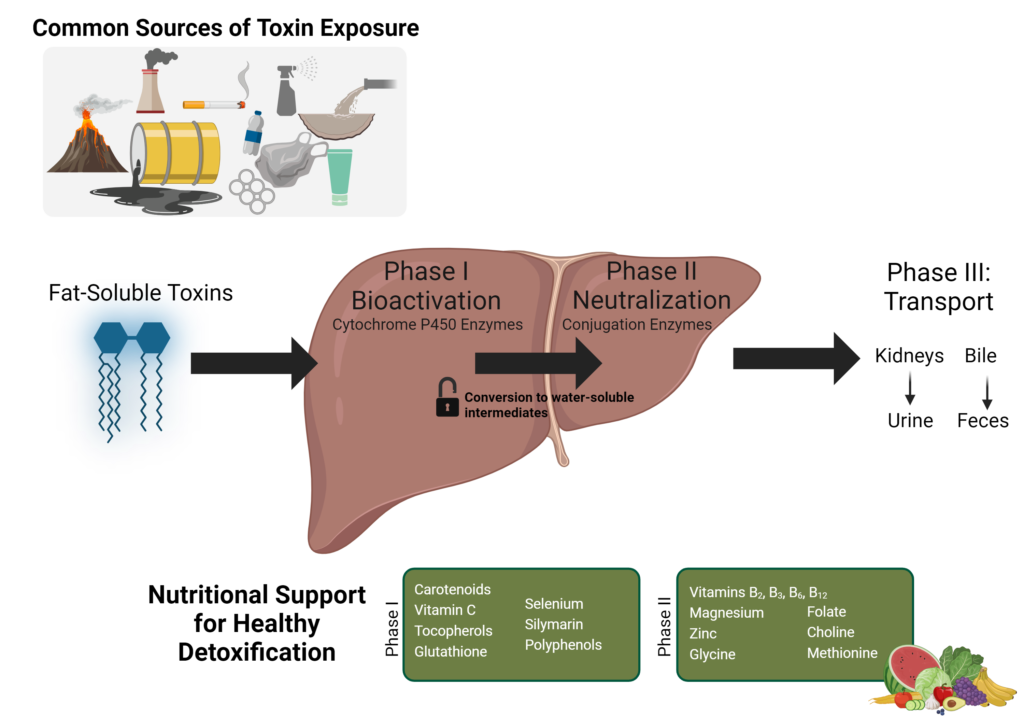
The majority of toxins are lipid-soluble molecules. It is difficult for the body to excrete lipid-soluble molecules, and these compounds can easily cross cell membranes, affecting cellular activities. These lipid-soluble toxins are stored mainly in adipose tissue and the central nervous system (CNS).
The body also has a complex, integrated system designed to convert lipid-soluble toxins to water-soluble molecules, after which they can be excreted through renal or biliary routes. This system is called the detoxification, or biotransformation, system, including Phase I and Phase II metabolizing enzymes and Phase III protein transporters.
The detoxification system converts lipid-soluble toxins to water-soluble molecules by conjugating (binding) the toxin to another molecule such as an amino acid, methyl group, or glutathione. However, most toxins do not naturally have a reactive site that enables them to bind to a conjugation molecule. Phase I enzymes create a reactive site on the toxin compound that enables conjugation.
THE BODY’S NATURAL DETOXIFICATION PROCESS
The human body is exposed to endogenous metabolic toxins every day. Toxin exposure can put pressure on the body’s natural metabolic detoxification capacity.

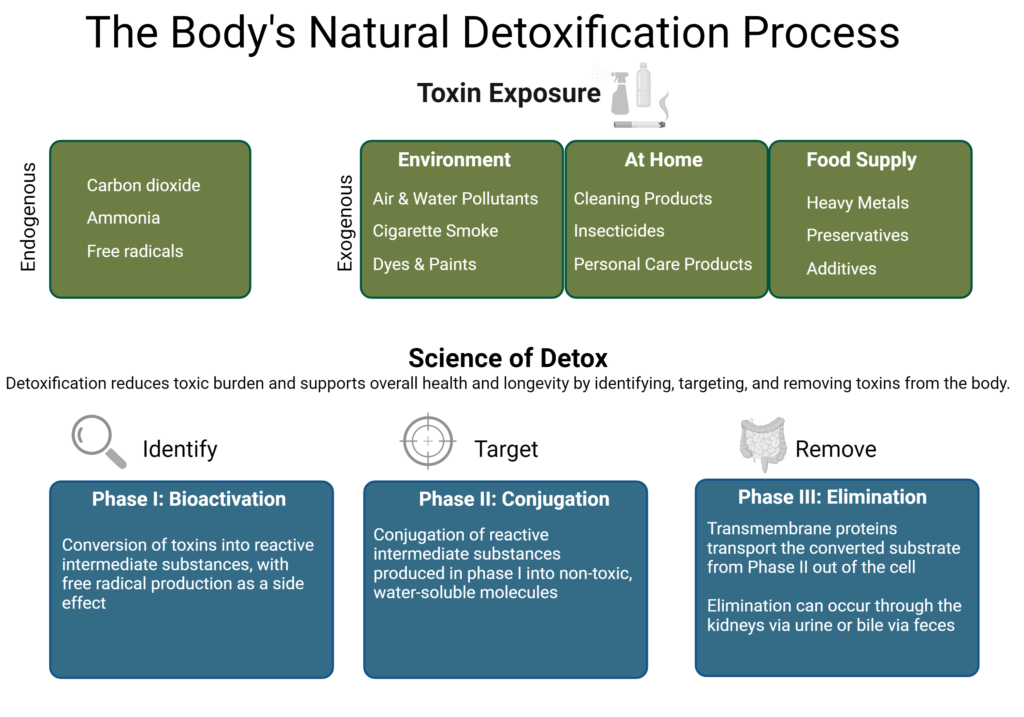
The Three Phases of Detoxification
The detoxification system is defined by three phase pathways:
- Phase I: Bioactivation
- Phase II: Conjugation
- Phase III: Transport
The detoxification system is highly dependent on proper nutrient support for optimal functioning. Nutritional support for the biotransformation system is extremely important for any detoxification program.1
Detox Phase I: Bioactivation
Phase I reactions are catalyzed by a number of different enzymes, primarily from the cytochrome P450 (CYP450) superfamily of enzymes. CYP450 enzymes conduct many chemical reactions (oxidation, reduction, hydrolysis, hydration, or dehalogenation) to add a reactive group (hydroxyl, carboxyl, or amino) to a toxin. Cruciferous vegetables such as Spanish black radish, broccoli (frozen or raw), cauliflower, fresh daikon radish sprouts, cabbage, and Brussels sprouts activate CYP450 enzymes.
The result of this reaction is the generation of a reactive site on the transformed toxin. This reactive site is very much like that of reactive oxygen species (ROS) and can readily bind to other molecules, such as DNA and proteins. Phase I activity converts toxin molecules into reactive intermediate substances (activated toxins), producing free radicals in the process. The reactive intermediate substances are considered more toxic than the parent toxin compounds, so they need to be neutralized quickly.
Protective nutrients with antioxidant properties that may help to mitigate oxidative stress, produced by phase I enzyme activity, include:
- Carotenes (Vitamin A)
- Ascorbic acid (Vitamin C)
- Tocopherols (Vitamin E) and selenium
- Copper, zinc, and manganese
- Coenzyme Q10,
- Thiols (found in garlic, onions, and cruciferous vegetables)
- Silymarin
- Bioflavonoids and polyphenols
Detox Phase II: Conjugation
Phase I activation results in the generation of reactive intermediates, which are often even more reactive — and potentially more toxic — than the parent molecule. Ideally, these reactive compounds should be converted to a non-toxic, water-soluble molecule at the site of production, as soon as possible. Conjugation of the reactive intermediates to water-soluble molecules is accomplished by Phase II conjugation enzymes, which consist of many enzyme superfamilies, including:
- Sulfotransferases (SULT)
- UDP-glucuronosyltransferases (UGT)
- Glutathione S-transferases (GST)
- N-acetyltransferases (NAT)6
Conjugation reactions not only require the water-soluble fraction that will be attached to the toxin—such as sulfate in the case of sulfation or glucuronic acid in the case of glucuronidation—but it also uses a large amount of energy in the form of adenosine triphosphate (ATP). In addition to energy repletion, Phase II reactions require an abundance of co-factors. Multiple nutrients and phytonutrients may help support Phase II reactions.
Table I. Phase II Conjugation Enzymes8
| Enzyme(s) | Reaction Name | Mechanism | Conjugated Compound(s) |
| UDP-Glucuronosyltransferases (UGTs) | Glucuronidation | Glucuronidation consists of transfer of the glucuronic acid component of uridine diphosphate glucuronic acid to a substrate (e.g. drugs, toxins, pollutants, estrogens, and glucocorticoids) | Glucuronic acid |
| Sulfotransferases (SULTs) | Sulfation (a.k.a. sulfonation or sulfurylation) | Sulfation consists of transfer of sulfuryl group to a substrate | Sulfuryl group |
| Glutathione S-transferases (GSTs) | Transfers a glutathione molecule to a substrate | Glutathione | |
| Amino acid transferases | Transfers amino acids of various types to a substrate | Amino acids used in phase II conjugation: arginine, cysteine, glutamine, glycine (most conjugated), ornithine, taurine | |
| N-Acetyl transferases | Transfers an acetyl group to a substrate | Acetyl group | |
| Methyltransferases (MTs) | Methylation | Transfers a methyl group from a methyl donor such as s-adenosylmethionine (SAMe) to a substrate | Methyl group |
Methyltransferase Detoxification Enzymes and Methylation Capacity
A cell’s methylation capacity is defined as the ratio of S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH). A ratio of less than four indicates an impaired methylation capacity, which could result from a decrease in SAM level (universal methyl donor) or an elevated SAH, a potent methyltransferase inhibitor. An increase in SAH level is detrimental to methylation pathway as it inhibits methyltransferases involved in methylation, and methyltransferases involved in Phase II enzyme and creatine synthesis, e.g. COMT and GAMT among others. Key nutrients for methylation support include:
- Folate
- Betaine
- Choline
- Methionine
- Vitamins B12, B6, B2, and B3
- Magnesium
- Zinc
- Molybdenum
Deficiency in any of these key nutrients would affect methylation capacity and hence ability to provide methyl groups to Phase II methyltransferases, affecting toxin removal, estrogen detox, and creatine synthesis.
It is prudent for patients with impaired methylation capacity to first improve their SAM:SAH ratio before introducing any detoxification programs. Lowering homocysteine may not be enough to restore methylation capacity. Patients should ensure that homocysteine levels as well as the SAM:SAH ratio is within the reference range. Many patients have healthy homocysteine levels while also having impaired SAM:SAH ratios (impaired methylation capacity).
Patients with SNPs in methylation pathway enzymes should not be discouraged from enrolling in detoxification regimens. Genes do not tell functional methylation capacity. Just because a patient has a SNP that might predispose them to a deficiency in methylation, it does not mean they actually have impaired methylation. In fact, they could have completely normal methylation.
Creatine and Methylation Capacity
Creatine in the form of creatine phosphate plays an important role in ATP regeneration. The human body excretes about two grams of creatine in the form of creatinine via urine daily. Humans replenish creatine stores via diet or de novo synthesis from glycine and arginine. Creatine is mainly found in red meat (muscle); it is scarce in other types of meat and is largely absent in plants.
Vegans and vegetarians rely on de novo synthesis of creatine and place high demand for glycine, arginine, and SAM, a methyl donor for the GAMT enzyme, the key enzyme in creatine synthesis). It is estimated that 75 percent of SAH produced in the human body is from GAMT activity. Studies showed that vegans and vegetarians tend to have higher homocysteine levels than the general population and hence, elevated SAH.
Glycine is the amino acid that is most frequently conjugated to toxins in humans. Glycine is also in high demand for creatine synthesis in vegetarians, vegans, and omnivores with low intake of red meat (creatine level is low in chicken and fish and is not enough to replenish creatine stores). During detoxification regimens, toxins stored in fat tissues are mobilized and activated by CYP450 enzymes to intermediate substances, leading to a demand for glycine by Phase II enzymes increases.
With poor nutrition, the supply of creatine and glycine from the diet may be inadequate. This may quickly lead to depletion of glycine reserves. Glycine would be required for de novo creatine synthesis and toxin conjugation (the demand for both pathways increases during detoxification). For every toxin removed, a molecule of glycine is lost forever from the body. This situation is more problematic in patients with elevated SAH, as increases in GAMT activity requires replenishment of creatine reserves.
Poor creatine in diet would further increase SAH levels, exacerbating methyltransferase inhibition and impairing toxin and estrogen detoxification. It is prudent for omnivores to cut down on red meat consumption but not to entirely eliminate red meat from their diet during detoxification. Vegans and vegetarians should be extra careful to consume a proper diet rich in glycine, arginine, cysteine, and key vitamins and minerals essential for methylation support especially vitamin B12.
Detox Phase III: Transport
Also known as the elimination phase, Phase III includes transmembrane-spanning proteins that transport substrate out of the cell. Most Phase III proteins are energy-dependent and utilize energy from ATP hydrolysis. Processed and water-soluble toxins are exported from the cell to the circulation for eventual elimination by the kidneys, or they are exported into bile and then excreted via the feces.
Human urine pH can range from 4.6 (acidic) to 8.0 (alkaline), and urinary pH may affect the elimination of toxins.7 For example, urine alkalinization increases the urine elimination of methyl chlorophenoxyproprionic (MCPP) acid and 2,4-dichlorophenoxyacetic acid (herbicides).3
In the event of acute poisoning or toxin overdose, alkalinization of urine to a pH of 7.5 or greater is a method for the enhanced elimination of toxins under acute medical settings.10 Clinical studies have shown that alkaline minerals (commonly found in fruit and vegetables) and plant-based dietary supplements increase urinary pH.11,12 Thus, progressive alkalinization of urine via dietary agents may assist metabolic detoxification by enhancing urinary excretion of weak acids. In addition, adequate water intake is essential to maintaining healthy kidney function and promoting urinary toxin excretion.
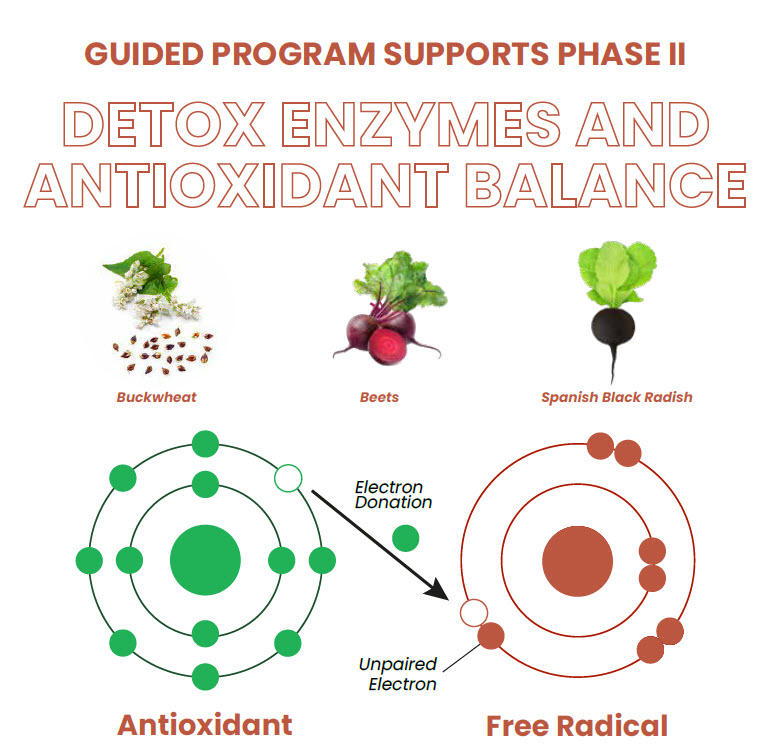
GUIDED PROGRAM SUPPORTS PHASE II DETOX ENZYMES AND ANTIOXIDANT BALANCE
As part of the Research Spotlight series on WholisticMatters, this infographic explores the effects of consuming a whole food-based nutritional supplement as part of a four-week guided metabolic detoxification program.
What is Potential Renal Acid Load?
Potential Renal Acid Load (PRAL) is a value calculated from a formula to assess the acidity of foods and diets developed by Thomas Remer and other researchers at the Research Institute of Child Nutrition Department of Nutrition and Health in Dortmund, Germany.
PRAL Formula
PRAL = 0.49 x protein (g) + 0.037 x phosphorus (mg) – 0.021 x potassium (mg) – 0.026 x magnesium (mg) – 0.013 x calcium (mg).13
Today, there is a general consensus that diet can markedly affect acid-base status and that a person’s acid load can be specifically manipulated by dietary means. In general, protein and cereal grains are metabolized to acidic residues, while fruits and vegetables are metabolized to alkaline residues. Current American diets are progressively more acidic in comparison to pre-agricultural diets (mean Net Acid Load of -88mEq/d versus +48mEq/d).14 With the decline in renal function that occurs with aging, older individuals are not able to excrete excess hydrogen ions, and they develop mild but slowly increasing metabolic acidosis.13
A study examined whether a plant-based dietary supplement, one marketed to increase alkalinity, impacts urinary pH.3 Using pH test strips, the urinary pH of 34 healthy men and women (33.9 +/- 1.57 y, 79.3 +/- 3.1 kg) was measured for seven days to establish a baseline urinary pH without supplementation.
After this initial baseline period, urinary pH was measured for an additional two weeks while participants ingested the plant-based nutritional supplement. At the end of the investigation, pH values at baseline and during the treatment period were compared to determine the efficacy of the supplement. Mean urinary pH statistically increased (p = 0.03) with the plant-based dietary supplement. Mean urinary pH was 6.07 +/- 0.04 during the baseline period and increased to 6.21 +/- 0.03 during the first week of treatment, and to 6.27 +/- 0.06 during the second week of treatment.3
The study concluded that supplementation with a plant-based dietary product for at least seven days increases urinary pH, potentially increasing the alkalinity of the body.15
Diets rich in fiber improve bowel movement and may expedite or facilitate the elimination of toxins from the body, providing support for Phase III of detoxification.14,15
Soluble dietary fibers facilitate urinary excretion of toxic elements.18 Several studies illustrate the impact of fiber on the detoxification process:
- Water-soluble dietary fibers from apple peels have shown significant binding capacities for toxic elements.19
- A study in elderly men on low fiber diets showed that consumption of isomalto-oligosaccharides (IMO or IO) effectively improved bowel movement, stool output, and microbial fermentation in the colon. Therefore, supplementation of IO into ordinary low fiber diets may be practical in relieving constipation in the elderly population.17
- A clinical study showed that oral administration of modified citrus pectin (MCP), a soluble fiber, significantly increases the urinary excretion of toxic metals in subjects with a “normal” body load of metals.20 MCP is a dietary supplement derived from the inner white pulp of citrus fruit peels. Citrus pectin is a complex polysaccharide that is a soluble fiber.
- A mixture of dietary fiber containing water-soluble dietary fibers from apple peels and water-insoluble dietary fibers from wheat bran and soybean-seed hull showed significant binding capacities for toxic elements (lead, mercury, and cadmium) and low binding capacity for the toxic anion ASO33+.19
- Dietary fiber (including fibers from apple) exerts a considerable effect on microbiota composition and on fecal short-chain fatty acid (SCFA) production in the elderly.21
- MCP has been shown to be an effective chelator of lead in children hospitalized with toxic lead levels.18
Did you know Wholistic Matters is powered by Standard Process? Learn more about Standard Process’ whole food-based nutrition philosophy and where to buy.
- Sears, M. E., Genuis, S. J. (2012). Environmental determinants of chronic disease and medical approaches: recognition, avoidance, supportive therapy, and detoxification. J Environ Public Health.
- Mostafalou, S., Abdollahi, M. (2013). Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol, 268 (2), 157-77.
- Jochmanova, I., Lazurova, Z., Rudnay, M., Bacova, I., Marekova, M., Lazurova, I. (2015). Environmental estrogen bisphenol A and autoimmunity. Lupus, 24 (4-5), 392-9.
- Liska, D., Lyon, M., Jones, D. S. (2006). Detoxification and biotransformational imbalances. Explore (NY), 2 (2), 122-40.
- Polanska, K., Jurewicz, J., Hanke, W. (2013). Review of current evidence on the impact of pesticides, polychlorinates biphenyls and selected metals on attention deficit / hyperactivity disorder in children. Int J Occup Med Environ Health, 26 (1), 16-38.
- Piomelli, S. (1981). Chemical toxicity of red cells. Environ Health Perspect, 39:65.
- Shetty, S.S., Deepthi, D., Harshitha, S., Sonkusare, S., Naik, P.B., Sucetha, K.N., et al. (2023) . Environmental pollutants and their effects on human health. Heliyon, 9(9):e19496.
- Hodges, R. E., Minich, D. M. (2015). Modulation of metabolic detoxification pathways using foods and food-derived components: a scientific review with clinical application. J Nutr Metab.
- Urine pH test. Retrieved from, https://www.nlm.nih.gov/medlineplus/ency/article/003583.htm
- Proudfoot, A. T., Krenzelok, E. P., Vale, J. A. (2004). Position paper on urine alkalinization. Journal of toxicology. Clinical toxicology, 42 (1), 1-26.
- Berardi, J. M., Logan, A. C., Rao, A. V. (2008). Plant based dietary supplement increases urinary pH. J Int Soc Sports Nutr, 5, 20.
- Konig, D., Muser, K., Dickhuth, H. H., Berg, A., Deibert, P. (2009). Effect of a supplement rich in alkaline minerals on acid-base balance in humans. Nutrition journal, 8, 23.
- Remer, T., Dimitriou, T., Manz, F. (2003). Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr, 77 (5), 1255-60.
- Sebastian, A., Frassetto, L. A., Sellmeyer, D. E., Merriam, R. L., Morris, R. C. (2002). Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr, 76 (6), 1308-16
- Berardi, J. M., Logan, A. C., Rao, A. V. (2008). Plant based dietary supplement increases urinary pH. J Int Soc Sports Nutr, 5, 20.
- Yen, C. H., Tseng, Y. H., Kuo, Y. W., Lee, M. C., Chen, H. L. (2011). Long-term supplementation of isomalto-oligosaccharides improved colonic microflora profile, bowel function, and blood cholesterol levels in constipated elderly people–a placebo-controlled, diet-controlled trial. Nutrition, 27 (4), 445-50.
- Chen, H. L., Lu, Y. H., Lin, J. J., Ko, L. Y. (2001). Effects of isomalto-oligosaccharides on bowel functions and indicators of nutritional status in constipated elderly men. J Am Coll Nutr, 20 (1), 44-9.
- Zhao, Z. Y., Liang, L., Fan, X., Yu, Z., Hotchkiss, A. T., Wilk, B. J., Eliaz, I. (2008). The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern Ther Health Med, 14 (4), 34-8.
- Zhang, N., Huang, C., Ou, S. (2011). In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J Hazard Mater, 186 (1), 236-9.
- Eliaz, I., Hotchkiss, A. T., Fishman, M. L., Rode, D. (2006). The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother Res, 20 (10), 859-64.
- Cuervo, A., Salazar, N., Ruas-Madiedo, P., Gueimonde, M., Gonzalez, S. (2013). Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutr Res, 33 (10), 811-6.







