The Role of mTOR in Aging and Longevity
mTOR (mammalian target of rapamycin or mechanistic target of rapamycin) is a protein in the body that is critical to cellular metabolism and has gained attention for its role in aging and longevity. mTOR is involved in nutrient sensing, which impacts cellular growth and proliferation, autophagy, mitochondrial function, and cellular senescence.1
History
Rapamycin, a natural product isolated from Streptomyces hygroscopicus, a soil bacterium, was discovered in the 1970s and found to exhibit many beneficial effects, including immunosuppressive, anti-cancer, and anti-fungal properties.1 These effects were mediated through the inhibition of a target protein: mammalian/mechanistic target of rapamycin or mTOR.1 Since its discovery, mTOR has been linked to a variety of processes associated with aging.1
Mechanism of Action
In humans, mTOR is encoded by a single gene, but the protein consists of two distinct complexes — mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2).1 They carry out different functions and also have unique sensitivities to rapamycin.1 mTORC1 is activated by extracellular and intracellular stimuli, including amino acids, hormones, growth factors, energetic stress, and oxygen.1 These stimuli lead to the initiation of mTOR-dependent anabolic processes, such as protein and nucleotide synthesis, while inhibiting autophagy. The net result is the stimulation of cellular growth and proliferation.1 mTORC2, on the other hand, is involved in other physiological pathways, including glucose and lipid metabolism, ion transport, and cytoskeleton and cell migration.2
mTOR regulates aging and longevity through its ability to act as a nutrient sensor. It coordinates a variety of nutrient and growth factor signals to coordinate basic cellular responses, including cellular growth, proliferation, and apoptosis.2 mTOR also regulates many hallmarks of aging, including autophagy, mitochondrial function, and cellular senescence.2 However, it is the inhibition of mTOR that helps extend the lifespan and support healthy aging processes.
In a variety of pre-clinical studies, inhibition of mTORC1 has been shown to extend the lifespan through several mechanisms.2 Because mTORC1 suppresses autophagy, inhibition of mTORC1 induces autophagy, which can help prevent the accumulation of damaged proteins and organelles in the cell, which naturally occurs with age.2 Additionally, senescent cells contribute to aging through the secretion of pro-inflammatory and pro-oxidant signals.2 mTORC1 drives many of the metabolic changes that occur in senescent cells; therefore, inhibiting mTOR pathways can help prevent metabolic stress, delay cellular senescence, and promote healthier aging.2 mTOR may also exert differential effects in specific tissues, such as the heart, adipose tissue, and skeletal muscle.1
Factors that Regulate mTOR
While pharmacological inhibitors of mTOR have been used in scientific studies, they can have significant side effects.2 Certain dietary interventions may inhibit mTOR to benefit health while avoiding negative side effects. For example, calorie restriction regulates several pathways that also interact with mTOR signaling pathways. Calorie restriction is a reduction of nutrient intake without malnutrition and is linked to mTOR through its role in nutrient sensing.2 As such, calorie restriction is thought to inhibit the activity of mTOR, although clinical studies are needed to confirm this conclusion.3
Other natural compounds may also directly or indirectly inhibit mTOR and related pathways.4 Curcumin, a compound in turmeric, can disrupt the formation of the mTOR-Raptor complex, an important rheostat that modulates mTORC1 activity.5,6 Other phytonutrients that have been found to inhibit the PI3K/Akt/mTOR pathway include resveratrol, epigallocatechin gallate (EGCG), genistein, and 3,3’-diindolylmethane (DIM), a breakdown product of indole-3-carbinol.7-11 Caffeine may also specifically inhibit mTORC1.12 Many of these compounds provide other health benefits due to their anti-inflammatory, antioxidant, and neuroprotective and anti-cancer properties. Together, the independent and synergistic effects may provide significant benefits to cells and organs during the aging process.
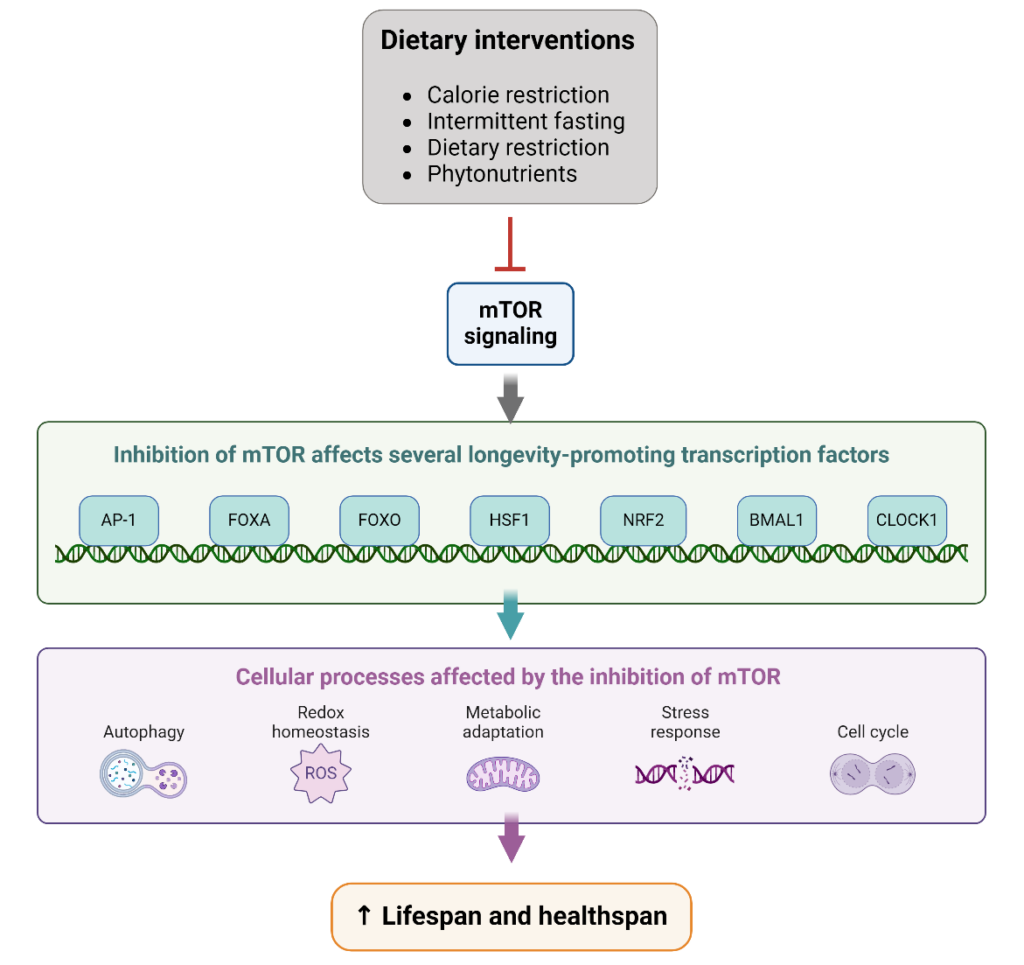
While thousands of cellular proteins exist throughout the body, mTOR continues to stand out as an important contributor to aging and longevity. Calorie restriction may be able to inhibit mTOR to promote healthy cellular function throughout the lifespan. Also, certain phytochemicals may also exert their beneficial effects through mTOR pathways and independent mechanisms that promote healthy inflammation and oxidative balance.
- Papadopoli, D., Boulay, K., Kazak, L., Pollak, M., Mallette, F.A., Topisirovic, I., et al. (2019). mTOR as a central regulator of lifespan and aging. F1000Res, 8:F1000.
- Weichart, T. (2018). mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology, 64(2):127.
- Tucci, P. (2012). Caloric restriction: is mammalian life extension linked to p53? Aging, 4(8):525.
- Zhou, H., Luo, Y., Huang, S. (2011). Updates of mTOR inhibitors. Anticancer Agents Med Chem, 10(7):571.
- Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res 2009;69:1000–1008
- Foster, K.G., Acosta-Jaquez, H.A., Romeo, Y., Ekim, B., Soliman, G.A., Carriere, A., et al. (2010). Regulation of mTOR complex 1 (mTORC1) by Raptor Ser863 and multisite phosphorylation. J Biol Chem, 285(1):80.
- Jiang, H., Shang, X., Wu, H., Gautam, S.C., Al-Holou, S., Li, C., et al. (2009). Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol,8:25.
- Zhang, Q., Kelly, A.P., Wang, L., French, S.W., Tang, X., Duong, H.S., et al. (2006). Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Invest Dermatol,126:2607.
- Anastasius, N., Boston, S., Lacey, M., Storing, N., Whitehead, S.A. (2009). Evidence that low-dose, long-term genistein treatment inhibits oestradiol-stimulated growth in MCF-7 cells by down-regulation of the PI3-kinase/Akt signalling pathway. J Steroid Biochem Mol Biol, 116:50.
- Nakamura, Y., Yogosawa, S., Izutani, Y., Watanabe, H., Otsuji, E., Sakai, T. (2009). A combination of indol-3- carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol Cancer,8:100.
- Kong, D., Banerjee, S., Huang, W., Li, Y., Wang, Z., Kim, H.R., et al. (2008). Mammalian target of rapamycin repression by 3,3′-diindolylmethane inhibits invasion and angiogenesis in platelet-derived growth factor-D-overexpressing PC3 cells. Cancer Res, 68:1927.
- Reinke, A., Chen, J.C., Aronova, S., Powers, T. (2006). Caffeine targets TOR complex I and provides evidence for a regulatory link between the FRB and kinase domains of Tor1p. J Biol Chem, 281:31616.







