Integrating Herbs for Neuroplasticity into Clinical Practice
Neuroplasticity, or the ability of the brain to respond to stimuli through adaptive and functional changes, is a central focus of research into therapies for addressing neurodegenerative disease, traumatic brain injury (TBI) and cognitive decline.
Diet, lifestyle factors, injury and the natural aging process can all impact neuroplasticity, often though the process of neuroinflammation, which compromises the function of the central and peripheral nervous systems.
Many botanicals show significant promise in mitigating neuroinflammation, promoting neuronal repair and improving overall brain health and neuroplasticity, effects which have been demonstrated in both pre-clinical and clinical studies.
This article focuses on four herbs for neuroplasticity with neuroprotective properties, Curcuma longa (Turmeric in the form of CurQfen® E40), Boswellia serrata (Boswellia with FenuMAT technology), Centella asiatica (Gotu Kola), and Bupleurum falcatum (Bupleurum) and the research supporting their integration into clinical practice for the improvement of cognitive function and brain health.
Abbreviations: Traumatic Brain Injury (TBI), Traditional Chinese Medicine (TCM), Brain-Derived Neurotrophic Factor (BDNF), 5-Lipoxygenase (5-LOX), nitric oxide (NO), Reactive Oxygen Species (ROS).
Introduction to Neuroplasticity
Also known as ‘neural plasticity’ or ‘brain plasticity,’ neuroplasticity refers to the adaptive and functional changes that occur within the brain and peripheral nervous system, both throughout the course of life and in response to various stimuli. Such stimuli include learning, repetitive tasks, the natural aging process and injury.1
Also known as ‘neural plasticity’ or ‘brain plasticity,’ neuroplasticity refers to the adaptive and functional changes that occur within the brain and peripheral nervous system, both throughout the course of life and in response to various stimuli. Such stimuli include learning, repetitive tasks, the natural aging process and injury.1
Mechanisms of Neuroplasticity
This capacity of the brain to adapt, particularly following a trauma such as stroke or traumatic brain injury (TBI), is dependent upon two primary mechanisms: functional reorganization and neuronal regeneration.
Functional reorganization is the concept that the brain can reorganize itself at a structural level. This process is dependent upon neuronal plasticity and is still being explored for the development of therapeutic interventions in the cases of neurodegenerative diseases and TBI.2
Neuronal regeneration is a complex process that involves both synaptic plasticity (changes in the strength of neuronal synapses) and synaptic communication to make experience-dependent and long-lasting changes in the strength of neuronal connections.3
These adaptive changes occurring at the synapse between neurons are the result of an intricate dynamic between neurotransmitter release, quantity and variety of postsynaptic receptors and involvement of surrounding neural structures.
Long-term potentiation, or strengthening of a synapse, and long-term depression, or weakening of a synapse, are two features of synaptic plasticity that have been studied primarily in the hippocampus, and are directly linked to learning, memory and longevity of cognitive function.4
A group of proteins collectively known as neurotrophins are considered to be crucial mediators of synaptic plasticity; in particular, Brain-Derived Neurotrophic Factor (BDNF) and Nerve Growth Factor (NGF) have received increasing attention for their ability to regulate synaptic plasticity. Deficits in these compounds have been linked to the pathogenesis of neurodegenerative diseases such as Parkinson’s Disease and Alzheimer’s Disease, as well as psychiatric disorders like depression and anxiety, making them a key target for the development of drugs and supplements to address brain health and function.5,6
Factors Influencing Neuroplasticity
Exercise, diet, sunlight exposure, and botanicals have all been linked to increased neurotrophin expression.7-9 Several factors, including exercise, learning, and neuromodulating compounds found in medications, herbs, and foods, can positively influence long-term potentiation and, therefore, improve neuronal structure and function.10-13
Acute and chronic stress, addictive drugs, injury, aging, metabolic dysfunction, inflammation, and neurodegenerative disease dysregulate synaptic plasticity, resulting in long-term depression of neuronal synapses, and can lead to a learned experience of anxiety, depressive and addictive behaviors as well as worsening the progression of neurodegeneration.14-17
Neuroinflammation: A Barrier to Neuroplasticity
Neuroinflammation is a primary driver of compromised neuroplasticity, leading to neurodegenerative diseases and cognitive impairment. Characterized by increased permeability of the Blood-Brain Barrier (BBB), activated microglial cells, and the release of inflammatory mediators (IL-1, IL-6, TNF-α), neuroinflammation results in neuronal damage, reduced production of protective neurotrophic factors, and, ultimately, neuronal signaling dysfunction.18-20
While neuroinflammation is obviously present in conditions related to the Central Nervous System (CNS), such as neurodegenerative diseases and TBI, there is also a strong connection between neuroinflammation and inflammation from peripheral organs, including the gut, lungs, and skin.
Autoimmune disorders, metabolic dysfunction, mental stress, lifestyle factors and even viral infections can all trigger the process of neuroinflammation.21
A growing number of conditions, both within and outside of the nervous system, have been linked to the presence of neuroinflammation, including ADHD, 22 Type 1 and 2 Diabetes,23 Premenstrual Dysphoric Disorder (PMDD),24 long COVID25 and sleep disorders.26
Mitigating peripheral and systemic inflammation through the management of potential triggering factors such as stress, obesity, and chronic pain, for example, are all strategies that can contribute to decreasing the presence of neuroinflammation to encourage overall brain health and function.
Botanicals and Neuroplasticity
Supporting neuroplasticity is necessary for both allowing neural networks to develop new capabilities and protecting their integrity over time.
Mood disorders and neuroinflammation are considered a bi-directional pathway, where the presence of one often leads to the development of the other.27
Many botanicals, foods, and medications that play a role in the management of anxiety and depression are being explored for their ability to mitigate neuroinflammatory processes that lead to neuronal damage and signaling dysfunction. These botanicals, many of which are categorized as ‘nootropic’ herbs, have long been used in traditional systems of medicine to support learning, memory, and mood.
Current research is helping to elucidate their potential modes of activity within the central nervous system, including enhancing mitochondrial function, reducing oxidative stress and inflammation, stimulating neurotransmitter expression, supporting BBB integrity and increasing neurotrophin production.9,28
The cognitive-supporting effects of these herbs are likely due to the synergistic effect of their complex phytochemistry, rather than being attributed to a single isolated compound, which supports the use of whole-plant preparations.
Additionally, both traditional use and modern evidence indicate that the majority of nootropic herbs need to be taken over a longer period of time, and within the appropriate therapeutic range, in order for their effects on cognitive function to be experienced or observed.
Key Herbs for Neuroplasticity
Curcuma longa (Turmeric in the form of CurQfen® E40)
Turmeric (Curcuma longa), a perennial member of the ginger family (Zingiberaceae) is renowned for its anti-inflammatory and antioxidant properties. It has been a core medicinal plant in Ayurveda and Traditional Chinese Medicine (TCM) for thousands of years, employed in the treatment of inflammatory disorders, gastrointestinal complaints, jaundice, menstrual difficulties, hematuria, hemorrhage, and colic, as well as topically to relieve pain and inflammation.29
Current research has demonstrated the antioxidant, hepatoprotective, neuroprotective, cardioprotective, anti-inflammatory, anticarcinogenic, and antimicrobial properties of both turmeric and its active constituent curcumin.30
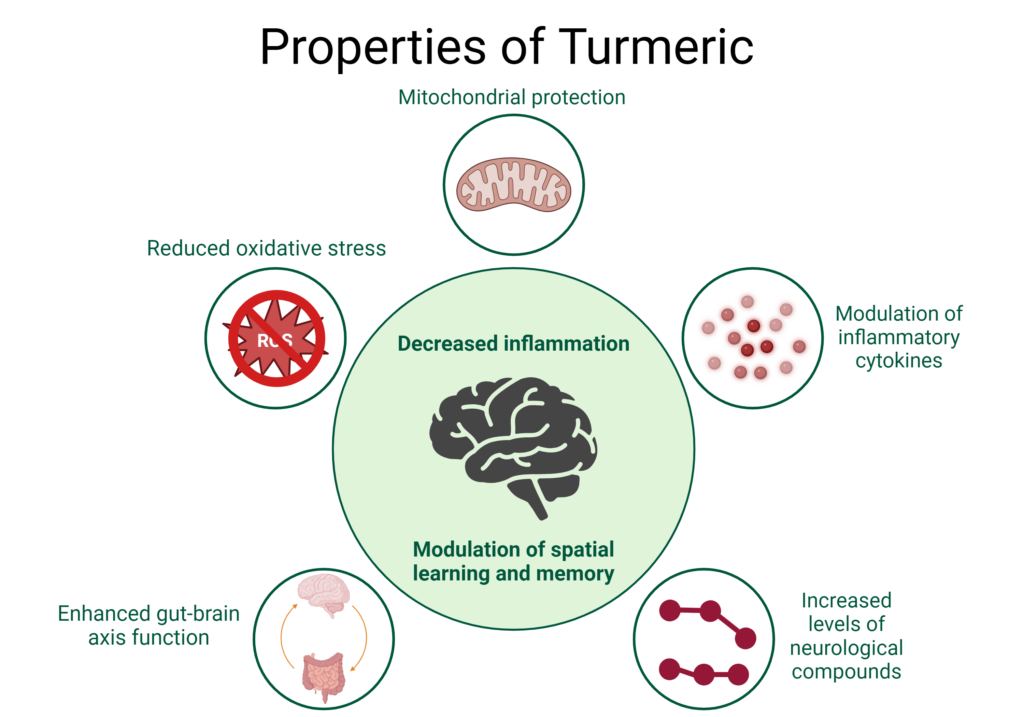
Mechanisms of Action
Turmeric and curcumin have displayed significant neuroprotective and neuroregenerative properties in pre-clinical research through a diversity of physiological pathways, including mitigating oxidative stress and protecting mitochondria, modulating inflammatory cytokines and improving the levels of key neurological regulatory compounds.
Excessive generation of Reactive Oxygen Species (ROS), as well as insufficient antioxidant capacity, results in DNA mutations in the mitochondria, damage to the mitochondrial respiratory chain, and alteration of membrane permeability, all of which have been linked to the development of neurodegenerative disease.31
Both turmeric and curcumin produce significant antioxidant activity, improving levels of glutathione, acetylcholinesterase, and catalase while reversing impairment of spatial learning and memory, indicating protective effects against ROS-induced mitochondrial dysfunction.31-33
In a model of nicotine-induced neuroinflammation curcumin demonstrated a protective effect against oxidative stress and apoptosis, attenuating increases of the pro-inflammatory cytokines IL-1β and TNF-α in the hippocampus and improving the activity of the activity of superoxide dismutase, glutathione peroxidase and glutathione reductase, mitigating the damage to the hippocampus observed with nicotine toxicity.34
This same study showed that curcumin also improved levels of two key neurological compounds: BDNF, one of the neurotrophins identified as having a regulatory role in synaptic plasticity, as well as phosphorylated CREB (pCREB) which regulates gene transcription related to plasticity, learning and memory.
Impact on Neuroinflammation and Mood Disorders
Numerous clinical trials have further demonstrated the profound anti-inflammatory properties of turmeric and curcumin, from mitigating oxidative stress and improving blood antioxidant capacity35 to decreasing levels of serum pro-inflammatory cytokines in individuals with metabolic syndrome.36
As systemic inflammation and oxidative stress have been directly linked to the pathogenesis of neuroinflammation and neurodegeneration, botanicals that attenuate these processes should be at the forefront of clinical practice when working with neuroinflammatory conditions.
Clinical trials have also demonstrated the benefits of curcumin in the treatment of mood disorders, ranging from anxiety to major depressive disorder.37-39
These studies have not only shown improvements in subjective measurements of mood but have also demonstrated improvements in key biomarkers of neuroinflammation, including evidence of decreases in inflammatory cytokines IL- 1β and TNF-α levels, increases plasma BDNF levels, and decreases in salivary cortisol concentration.39
Recent studies have identified the existence of a gut-brain axis, and the role of this complex network in the regulation of neurotransmitters, immunity and microbiome composition.40 This bidirectional axis serves as a communication highway between the central and enteric nervous systems and has gained attention as a potential target for drug development in the treatment of both neurological and gastrointestinal disorders.
Regulation of intestinal inflammation, improving gut barrier function and improving microbiome composition have all been demonstrated in clinical trials using curcumin, highlighting the role of this compound in modulating the functioning of the gut-brain axis.41,42
Bioavailability Challenges and Solutions
A key limiting factor for the therapeutic efficacy of turmeric formulations is the relatively poor bioavailability of curcuminoids due to low absorption by the small intestine and extensive metabolism by the liver.43
CurQfen® E40 technology is used to drastically increase the bioavailability of curcumin and has been shown to exhibit improved blood brain barrier (BBB) permeability and brain tissue distribution.44 \Clinically, the curcumin-galactomannan formulation has significantly improved both the Mini-Mental State Examination and Geriatric Locomotive Function Scale in patients with Alzheimer’s Disease, as well as improving serum concentration of key neurological and inflammatory biomarkers including BDNF, tau protein, IL-6 and TNF-α.45
Boswellia serrata (Boswellia with FenuMAT technology)
Chronic inflammation in the brain can contribute to the deterioration of neurons and loss of synaptic plasticity. These inflammatory processes are directly linked to the pathogenesis of numerous diseases, including Alzheimer’s and Parkinson’s Disease, as well as in the clinical and functional outcomes associated with TBI.46,47
Boswellia (Boswellia serrata), also known as Indian frankincense, has been used for centuries for its anti-inflammatory properties, including as an anti-arthritic medicine in the Ayurvedic tradition.48
Mechanisms of Action
The gummy oleoresin extracted from the tree contains numerous compounds, including boswellic acid, that have demonstrated anti-inflammatory activity both in vitro and in vivo.
In vitro administration of Boswellia extracts and isolated 3-acetyl-11-keto-β-boswellic acid (AKBA) directly inhibits 5-lipoxygenase (5-LOX),49-51 a key enzyme in the synthesis of pro-inflammatory leukotrienes, that has emerged as a target against TBI, brain tumors and neurodegenerative diseases.52,53
Other mechanisms by which Boswellia and its compounds mitigate inflammation in vitro include inhibition of tumor necrosis factor-alpha (TNF-alpha), interleukin-1beta (IL-1beta), nitric oxide (NO), mitogen activated protein (MAP) kinases and nuclear factor-kappa B (NF-kB).54,55
Clinical Evidence and Cognitive Benefits
In animal studies, Boswellia extracts have exerted potent anti-inflammatory and antioxidant activity that protects dopaminergic neurons and improves motor impairments and behavioral characteristics in models of neurodegenerative diseases.56,57
Several clinical studies have demonstrated the anti-inflammatory effects of Boswellia, particularly in the case of osteoarthritis where it has significantly improved functional ability and reduced pain in as little as 30 days of use.58-60
While limited in number, clinical studies further demonstrate substantial cognitive improvements in patients with neuroinflammatory conditions, including TBI, diffuse axonal injury, and Alzheimer’s,61-63 and even reduce cerebral edema in patients irradiated for brain tumors.64
Enhancing Bioavailability with FenuMAT Technology
Standardizing botanicals to obtain higher amounts of their active compounds as well as improve delivery and bioavailability is a crucial aspect of achieving desired therapeutic outcomes, particularly in the case of lipophilic compounds like boswellic acids.
FenuMAT Technology for Boswellia preparations enhances the solubility and bioavailability of boswellic acids, making them more effective.65 FenuMAT employs the use of fenugreek galactomannans, a natural prebiotic fiber, which is ideal for enhancing absorption and systemic circulation, as well as a slow release, stability and permeability.
Gotu kola
Gotu kola (Centella asiatica) is a creeping ground cover plant in the Apiaceae family that has been used for thousands of years in TCM and Ayurveda. In Ayurveda it is known as a ‘Medhya Rasayana,’ or an herb that improves memory and intellect, as well as supports nourishment, health, immunity and longevity.66
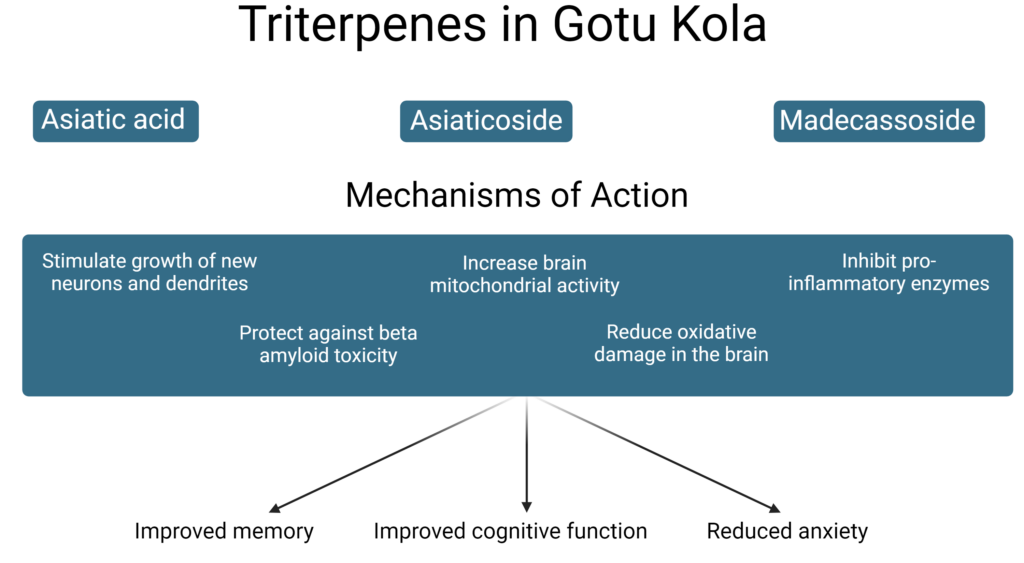
Neuroprotective Compounds and Actions
Contemporary research has shown that certain compounds in gotu kola, including asiaticoside, are able to impact both the central and peripheral nervous system through multiple mechanisms.
On a molecular level, gotu kola has demonstrated the ability to modulate several of the neurotransmitters and neurotrophins integral to brain functioning.
One preclinical study exploring the use of gotu kola in epileptic disorders showed that a water extract of the herb was able to increase acetylcholine, a key neurotransmitter for voluntary movement as well as learning, attention and memory, highlighting the herb’s potential for use in treatment of neurodegenerative disorders as well as cognitive enhancement.67
BDNF, recognized for its role in regulating synaptic plasticity, is also significantly upregulated by both the whole plant extract of gotu kola as well as isolated asiaticoside.68,69
Neurotropic and neuroprotective activities of gotu kola have been demonstrated in several in vitro models and are associated primarily with the triterpene compounds asiatic acid, asiaticoside and madecassoside. These phytochemicals stimulate the growth of new neurons and dendrites,70 protect against beta amyloid toxicity,71 increase brain mitochondrial activity,72 reduce oxidative damage in the brain,73 and inhibit the pro-inflammatory enzyme phospholipase A2 (PLA2).74
Increased oxidative stress, inflammation and PLA2 alongside decreased mitochondrial activity are common features of many neurodegenerative diseases as well as traumatic brain injury. These neuroprotective and neuroregenerative activities are further seen in animal models of stroke and neurodegeneration where gotu kola administration has ameliorated neuronal damage and improved memory and learning flexibility deficits.75-77
Effects on Stress and Anxiety
Gotu kola has also been shown to reduce stress and anxiety, two factors which contribute to long-term depression of synaptic plasticity. Research suggests its anxiolytic properties may be due to GABAergic effects.
Aqueous and ethanolic extracts of the herb have been shown to significantly increase the activity of glutamate decarboxylase, a key enzyme in the synthesis of GABA.78
Gotu kola in combination with turmeric was shown to increase levels of BDNF and the cell survival regulator mTOR, indicating the formulation shows promise in improving impairments in neuroplastic mechanisms, memory and cognitive abilities.79
Clinical studies further support the traditional use and preclinical evidence of the ability of gotu kola to improve memory and cognitive function and reduce anxiety.
In patients with anxiety, administration of gotu kola extract significantly attenuated anxiety-related disorders as well as significantly reduced stress phenomenon and its correlated depression. Additionally, gotu kola extract significantly improved the willingness for adjustment and cognition.80
Elderly individuals taking gotu kola extract for just two months showed enhanced working memory and increased EEG measurements for amplitudes related to auditory, visual, somatic, behavioral and cognitive tasks, as well as improvements in self-rated mood.81
Bupleurum falcatum (Bupleurum)
The root of bupleurum (Bupleurum falcatum) has been used in TCM and traditional Korean medicine for over 2,000 years for the treatment of chronic liver disorders, fevers, infection and inflammation.82
The majority of contemporary research into the therapeutic effects of bupleurum is centered around the saikosaponin compounds, which have demonstrated antiviral, anti-inflammatory, hepatoprotective, antitumor, anticonvulsant and neuroprotective properties in vitro.83
Potential mechanisms of action for the neuroprotective effects of bupleurum include the inhibition of NF-κB-mediated inflammatory pathways, reduction of oxidative stress and mitigation of protein compounds connected to neurodegenerative diseases.
In vitro and in vivo studies have shown that both the root extract and isolated saikosaponins significantly decrease levels of NO, iNOS, reactive oxygen species and the inflammatory cytokines IL-6, IL-1β, and TNF-α in models of neuroinflammation and neurodegeneration.84,85
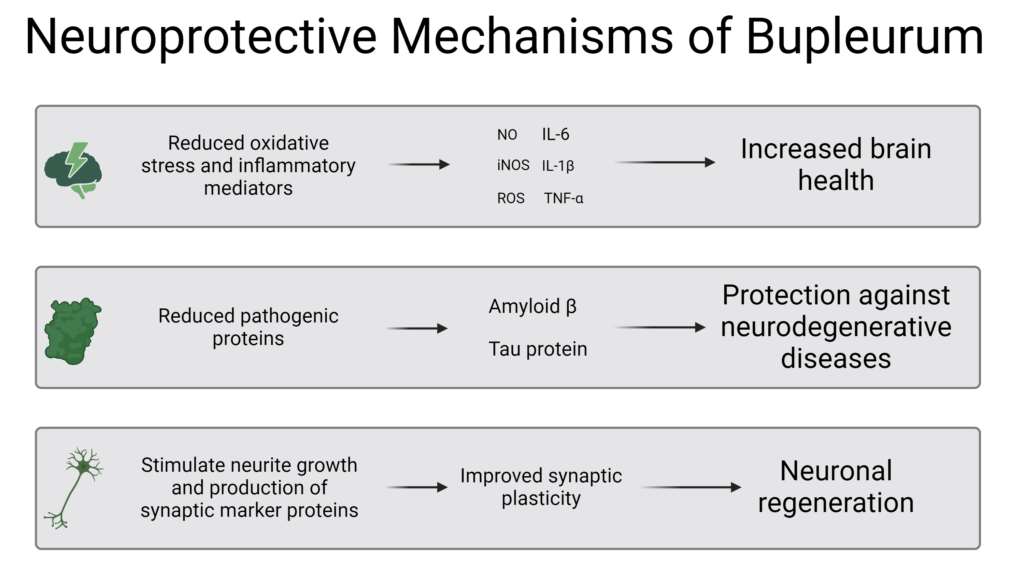
Mechanisms of Action
Saikosaponin C in particular has been shown to mitigate two key inflammatory proteins implicated in the pathogenesis of Alzheimer’s Disease, amyloid beta (Aβ) and tau, as well as stimulation of neurite growth and increased production of synaptic marker proteins, indicating improved synaptic plasticity.86
Historically bupleurum was used in the treatment of depression, the pathogenesis of which has been strongly linked with neuroinflammatory processes. This traditional use has been supported by pre-clinical research showing that saikosaponin compounds both reverse depressive-like behaviors and mitigate neural damage in models of depression.87
In TCM and Korean traditional medicine, bupleurum was used extensively to treat chronic liver diseases.
Liver disorders such as Non-Alcoholic Fatty Liver Disease (NAFLD) that interrupt the gut-brain axis and impact the liver’s ability to regulate glucose metabolism and detoxification have a profound impact on cognitive function,88 which opens a unique door for hepatoprotective and neuroprotective herbs like bupleurum to modulate this communication network to improve both liver function and cognition.
Clinical Evidence of Bupleurum’s Neuroprotective Effects
While clinical studies exploring bupleurum and saikosaponins in neurological disorders are limited, the preclinical research strongly supports the use of the root in modulating numerous pathways associated with neurodegenerative diseases, TBI and age-related cognitive decline.
These effects have been demonstrated in one clinical trial exploring the use of bupleurum in depression patients, which showed that after three months of treatment the patients receiving bupleurum had greater improvement in depression symptoms, anxiety symptoms and general functioning when compared to placebo, and showed serum increases in two key neurotrophic factors related to synaptic plasticity, BDNF and Nerve Growth Factor (NGF).89
Summary and Clinical Recommendations
Collectively, turmeric, Boswellia, gotu kola and bupleurum provide a multitude of actions to decrease neuroinflammation and enhance neuroplasticity.
Integrating these herbs into clinical practice offers a natural and effective approach for the management of the variety of disorders connected to neuronal function, including neurodevelopmental disorders, mood and psychiatric disorders, impaired cognition, dementia, neurodegenerative disease and brain injury.
While each herb has unique properties that contribute to their neurological effects, the synergistic use of botanicals has often been recommended by phytotherapists to increase therapeutic efficacy, and creates the opportunity for encouraging wide-ranging, systemic health benefits.
Careful consideration of dosages and patient-specific factors, as well as incorporating nutritional and lifestyle modifications, will maximize the benefits of these herbs for neuroplasticity as well as support safe usage across diverse patient populations.
- Gulyaeva NV. Molecular Mechanisms of Neuroplasticity: An Expanding Universe. Biochemistry (Mosc). Mar 2017;82(3):237-242. doi:10.1134/s0006297917030014
- Puderbaugh M, Emmady PD. Neuroplasticity. StatPearls. 2024.
- Mateos-Aparicio P, Rodríguez-Moreno A. The Impact of Studying Brain Plasticity. Front Cell Neurosci. 2019;13:66. doi:10.3389/fncel.2019.00066
- Bin Ibrahim MZ, Benoy A, Sajikumar S. Long-term plasticity in the hippocampus: maintaining within and ‘tagging’ between synapses. Febs j. Apr 2022;289(8):2176-2201. doi:10.1111/febs.16065
- Colucci-D’Amato L, Speranza L, Volpicelli F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int J Mol Sci. Oct 21 2020;21(20)doi:10.3390/ijms21207777
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. May 2013;138(2):155-75. doi:10.1016/j.pharmthera.2013.01.004
- Molendijk ML, Haffmans JP, Bus BA, et al. Serum BDNF concentrations show strong seasonal variation and correlations with the amount of ambient sunlight. PLoS One. 2012;7(11):e48046. doi:10.1371/journal.pone.0048046
- Gyorkos A, Baker MH, Miutz LN, Lown DA, Jones MA, Houghton-Rahrig LD. Carbohydrate-restricted Diet and Exercise Increase Brain-derived Neurotrophic Factor and Cognitive Function: A Randomized Crossover Trial. Cureus. Sep 9 2019;11(9):e5604. doi:10.7759/cureus.5604
- Sangiovanni E, Brivio P, Dell’Agli M, Calabrese F. Botanicals as Modulators of Neuroplasticity: Focus on BDNF. Neural Plast. 2017;2017:5965371. doi:10.1155/2017/5965371
- Vints WAJ, Levin O, Fujiyama H, Verbunt J, Masiulis N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front Neuroendocrinol. Jul 2022;66:100993. doi:10.1016/j.yfrne.2022.100993
- Bliss TV, Cooke SF. Long-term potentiation and long-term depression: a clinical perspective. Clinics (Sao Paulo). 2011;66 Suppl 1(Suppl 1):3-17. doi:10.1590/s1807-59322011001300002
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. Aug 25 2006;313(5790):1093-7. doi:10.1126/science.1128134
- Fadó R, Molins A, Rojas R, Casals N. Feeding the Brain: Effect of Nutrients on Cognition, Synaptic Function, and AMPA Receptors. Nutrients. Oct 5 2022;14(19)doi:10.3390/nu14194137
- Rygvold TW, Hatlestad-Hall C, Elvsåshagen T, Moberget T, Andersson S. Long-term potentiation-like visual synaptic plasticity is negatively associated with self-reported symptoms of depression and stress in healthy adults. Frontiers in Human Neuroscience. 2022;16:867675.
- Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Molecular psychiatry. 2020;25(3):530-543.
- Appelbaum LG, Shenasa MA, Stolz L, Daskalakis Z. Synaptic plasticity and mental health: methods, challenges and opportunities. Neuropsychopharmacology. 2023/01/01 2023;48(1):113-120. doi:10.1038/s41386-022-01370-w
- Mora F. Successful brain aging: plasticity, environmental enrichment, and lifestyle. Dialogues Clin Neurosci. Mar 2013;15(1):45-52. doi:10.31887/DCNS.2013.15.1/fmora
- Tian Z, Ji X, Liu J. Neuroinflammation in Vascular Cognitive Impairment and Dementia: Current Evidence, Advances, and Prospects. Int J Mol Sci. Jun 2 2022;23(11)doi:10.3390/ijms23116224
- Hong H, Kim BS, Im HI. Pathophysiological Role of Neuroinflammation in Neurodegenerative Diseases and Psychiatric Disorders. Int Neurourol J. May 2016;20(Suppl 1):S2-7. doi:10.5213/inj.1632604.302
- Gorji A. Neuroinflammation: The Pathogenic Mechanism of Neurological Disorders. Int J Mol Sci. May 20 2022;23(10)doi:10.3390/ijms23105744
- Sun Y, Koyama Y, Shimada S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front Aging Neurosci. 2022;14:903455. doi:10.3389/fnagi.2022.903455
- Dunn GA, Nigg JT, Sullivan EL. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol Biochem Behav. Jul 2019;182:22-34. doi:10.1016/j.pbb.2019.05.005
- Rom S, Zuluaga-Ramirez V, Gajghate S, et al. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol Neurobiol. Mar 2019;56(3):1883-1896. doi:10.1007/s12035-018-1195-5
- Bannister E. There is increasing evidence to suggest that brain inflammation could play a key role in the aetiology of psychiatric illness. Could inflammation be a cause of the premenstrual syndromes PMS and PMDD? Post Reproductive Health. 2019;25(3):157-161. doi:10.1177/2053369119875386
- Klein R, Soung A, Sissoko C, et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Res Sq. Oct 29 2021;doi:10.21203/rs.3.rs-1031824/v1
- Gnoni V, Ilic K, Drakatos P, et al. Obstructive sleep apnea and multiple facets of a neuroinflammatory response: a narrative review. J Thorac Dis. Feb 2022;14(2):564-574. doi:10.21037/jtd-21-1231
- Beurel E, Toups M, Nemeroff CB. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron. Jul 22 2020;107(2):234-256. doi:10.1016/j.neuron.2020.06.002
- Qiao J, Wang C, Chen Y, et al. Herbal/Natural Compounds Resist Hallmarks of Brain Aging: From Molecular Mechanisms to Therapeutic Strategies. Antioxidants. 2023;12(4):920.
- Curcuma longa (turmeric). Monograph. Altern Med Rev. Sep 2001;6 Suppl:S62-6.
- Sharifi-Rad J, Rayess YE, Rizk AA, et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front Pharmacol. 2020;11:01021. doi:10.3389/fphar.2020.01021
- Sathyabhama M, Priya Dharshini LC, Karthikeyan A, Kalaiselvi S, Min T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules. Oct 1 2022;12(10)doi:10.3390/biom12101405
- Banji D, Banji OJF, Srinivas K. Neuroprotective Effect of Turmeric Extract in Combination with Its Essential Oil and Enhanced Brain Bioavailability in an Animal Model. Biomed Res Int. 2021;2021:6645720. doi:10.1155/2021/6645720
- Yuliani S, Mustofa, Partadiredja G. The neuroprotective effects of an ethanolic turmeric (Curcuma longa L.) extract against trimethyltin-induced oxidative stress in rats. Nutr Neurosci. Nov 2019;22(11):797-804. doi:10.1080/1028415x.2018.1447267
- Motaghinejad M, Motevalian M, Fatima S, Faraji F, Mozaffari S. The Neuroprotective Effect of Curcumin Against Nicotine-Induced Neurotoxicity is Mediated by CREB-BDNF Signaling Pathway. Neurochem Res. Oct 2017;42(10):2921-2932. doi:10.1007/s11064-017-2323-8
- Takahashi M, Suzuki K, Kim HK, et al. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int J Sports Med. Jun 2014;35(6):469-75. doi:10.1055/s-0033-1357185
- Panahi Y, Hosseini MS, Khalili N, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed Pharmacother. Aug 2016;82:578-82. doi:10.1016/j.biopha.2016.05.037
- Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD. Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord. 2014;167:368-75. doi:10.1016/j.jad.2014.06.001
- Esmaily H, Sahebkar A, Iranshahi M, et al. An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin J Integr Med. May 2015;21(5):332-8. doi:10.1007/s11655-015-2160-z
- Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J Clin Psychopharmacol. Aug 2015;35(4):406-10. doi:10.1097/jcp.0000000000000352
- Yan M, Man S, Sun B, et al. Gut liver brain axis in diseases: the implications for therapeutic interventions. Signal Transduction and Targeted Therapy. 2023/12/06 2023;8(1):443. doi:10.1038/s41392-023-01673-4
- Pivari F, Mingione A, Piazzini G, et al. Curcumin Supplementation (Meriva(®)) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients. Jan 5 2022;14(1)doi:10.3390/nu14010231
- Szymanski MC, Gillum TL, Gould LM, Morin DS, Kuennen MR. Short-term dietary curcumin supplementation reduces gastrointestinal barrier damage and physiological strain responses during exertional heat stress. J Appl Physiol (1985). Feb 1 2018;124(2):330-340. doi:10.1152/japplphysiol.00515.2017
- Dei Cas M, Ghidoni R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients. Sep 8 2019;11(9)doi:10.3390/nu11092147
- Im K, Maliakel A, G G, Kumar D, Maliakel B, Kuttan R. Improved blood–brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. Journal of Functional Foods. 2015/04/01/ 2015;14:215-225. doi:https://doi.org/10.1016/j.jff.2015.01.049
- Das SS, Gopal PM, Thomas JV, et al. Influence of CurQfen®-curcumin on cognitive impairment: a randomized, double-blinded, placebo-controlled, 3-arm, 3-sequence comparative study. Frontiers in Dementia. 2023;2:1222708.
- Postolache TT, Wadhawan A, Can A, et al. Inflammation in Traumatic Brain Injury. J Alzheimers Dis. 2020;74(1):1-28. doi:10.3233/jad-191150
- Giri PM, Banerjee A, Ghosal A, Layek B. Neuroinflammation in Neurodegenerative Disorders: Current Knowledge and Therapeutic Implications. International Journal of Molecular Sciences. 2024;25(7):3995.
- Boswellia serrata. Altern Med Rev. Aug 1998;3(4):306-7.
- Siemoneit U, Pergola C, Jazzar B, et al. On the interference of boswellic acids with 5-lipoxygenase: mechanistic studies in vitro and pharmacological relevance. Eur J Pharmacol. Mar 15 2009;606(1-3):246-54. doi:10.1016/j.ejphar.2009.01.044
- Ammon H, Mack T, Singh G, Safayhi H. Inhibition of leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of Boswellia serrata. Planta medica. 1991;57(03):203-207.
- Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon H. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. Journal of Pharmacology and Experimental Therapeutics. 1992;261(3):1143-1146.
- Zhang L, Zhang WP, Hu H, et al. Expression patterns of 5-lipoxygenase in human brain with traumatic injury and astrocytoma. Neuropathology. Apr 2006;26(2):99-106. doi:10.1111/j.1440-1789.2006.00658.x
- Yan M, Zhang S, Li C, et al. 5-Lipoxygenase as an emerging target against age-related brain disorders. Ageing Research Reviews. 2021/08/01/ 2021;69:101359. doi:https://doi.org/10.1016/j.arr.2021.101359
- Gayathri B, Manjula N, Vinaykumar K, Lakshmi B, Balakrishnan A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFα, IL-1β, NO and MAP kinases. International immunopharmacology. 2007;7(4):473-482.
- Moussaieff A, Shohami E, Kashman Y, et al. Incensole acetate, a novel anti-inflammatory compound isolated from Boswellia resin, inhibits nuclear factor-κB activation. Molecular Pharmacology. 2007;72(6):1657-1664.
- Doaee P, Rajaei Z, Roghani M, Alaei H, Kamalinejad M. Effects of Boswellia serrata resin extract on motor dysfunction and brain oxidative stress in an experimental model of Parkinson’s disease. Avicenna J Phytomed. May-Jun 2019;9(3):281-290.
- Minj E, Upadhayay S, Mehan S. Nrf2/HO-1 Signaling Activator Acetyl-11-keto-beta Boswellic Acid (AKBA)-Mediated Neuroprotection in Methyl Mercury-Induced Experimental Model of ALS. Neurochem Res. Nov 2021;46(11):2867-2884. doi:10.1007/s11064-021-03366-2
- Majeed M, Majeed S, Narayanan NK, Nagabhushanam K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother Res. May 2019;33(5):1457-1468. doi:10.1002/ptr.6338
- Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee–a randomized double blind placebo controlled trial. Phytomedicine. Jan 2003;10(1):3-7. doi:10.1078/094471103321648593
- Karlapudi V, Sunkara KB, Konda PR, Sarma KV, Rokkam MP. Efficacy and Safety of Aflapin®, a Novel Boswellia Serrata Extract, in the Treatment of Osteoarthritis of the Knee: A Short-Term 30-Day Randomized, Double-Blind, Placebo-Controlled Clinical Study. J Am Nutr Assoc. Feb 2023;42(2):159-168. doi:10.1080/07315724.2021.2014370
- Karima S, Aghamollaii V, Mahmoodi Baram S, et al. Boswellic Acids Improve Clinical Cognitive Scores and Reduce Systemic Inflammation in Patients with Mild to Moderate Alzheimer’s Disease. J Alzheimers Dis. 2023;94(1):359-370. doi:10.3233/jad-221026
- Meshkat S, Mahmoodi Baram S, Rajaei S, et al. Boswellia serrata extract shows cognitive benefits in a double-blind, randomized, placebo-controlled pilot clinical trial in individuals who suffered traumatic brain injury. Brain Injury. 2022/03/21 2022;36(4):553-559. doi:10.1080/02699052.2022.2059816
- Moein P, Abbasi Fard S, Asnaashari A, et al. The effect of Boswellia Serrata on neurorecovery following diffuse axonal injury. Brain Inj. 2013;27(12):1454-60. doi:10.3109/02699052.2013.825009
- Kirste S, Treier M, Wehrle SJ, et al. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: a prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer. Aug 15 2011;117(16):3788-95. doi:10.1002/cncr.25945
- Abhilash MB, Kumar D, Deepti A, et al. Enhanced absorption ofcurcuminoidsand 3-Acetyl-11-keto-β-boswellic acid from fenugreek galactomannan hydrogel beadlets: A natural approach to the co-delivery of lipophilic phytonutrients. Journal of Functional Foods. 2021/04/01/ 2021;79:104405. doi:https://doi.org/10.1016/j.jff.2021.104405
- Kulkarni R, Girish KJ, Kumar A. Nootropic herbs (Medhya Rasayana) in Ayurveda: An update. Pharmacogn Rev. Jul 2012;6(12):147-53. doi:10.4103/0973-7847.99949
- Visweswari G, Prasad KS, Chetan PS, Lokanatha V, Rajendra W. Evaluation of the anticonvulsant effect of Centella asiatica (gotu kola) in pentylenetetrazol-induced seizures with respect to cholinergic neurotransmission. Epilepsy Behav. Mar 2010;17(3):332-5. doi:10.1016/j.yebeh.2010.01.002
- Sari DCR, Arfian N, Tranggono U, Setyaningsih WAW, Romi MM, Emoto N. Centella asiatica (Gotu kola) ethanol extract up-regulates hippocampal brain-derived neurotrophic factor (BDNF), tyrosine kinase B (TrkB) and extracellular signal-regulated protein kinase 1/2 (ERK1/2) signaling in chronic electrical stress model in rats. Iran J Basic Med Sci. Oct 2019;22(10):1218-1224. doi:10.22038/ijbms.2019.29012.7002
- Luo L, Liu X-L, Mu R-H, et al. Hippocampal BDNF signaling restored with chronic asiaticoside treatment in depression-like mice. Brain Research Bulletin. 2015/05/01/ 2015;114:62-69. doi:https://doi.org/10.1016/j.brainresbull.2015.03.006
- Mohandas Rao KG, Muddanna Rao S, Gurumadhva Rao S. Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Evid Based Complement Alternat Med. Sep 2006;3(3):349-57. doi:10.1093/ecam/nel024
- Chen C-L, Tsai W-H, Chen C-J, Pan T-M. Centella asiatica extract protects against amyloid β1–40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. Journal of Traditional and Complementary Medicine. 2016;6(4):362-369.
- Gray NE, Sampath H, Zweig JA, Quinn JF, Soumyanath A. Centella asiatica attenuates amyloid-β-induced oxidative stress and mitochondrial dysfunction. Journal of Alzheimer’s Disease. 2015;45(3):933-946.
- Doknark S, Mingmalairak S, Vattanajun A, Tantisira B, Tantisira MH. Study of ameliorating effects of ethanolic extract of Centella asiatica on learning and memory deficit in animal models. Journal of the Medical Association of Thailand= Chotmaihet Thangphaet. 2014;97:S68-76.
- Defillipo PP, Raposo AH, Fedoce AG, et al. Inhibition of cPLA2 and sPLA2 activities in primary cultures of rat cortical neurons by Centella asiatica water extract. Nat Prod Commun. Jul 2012;7(7):841-3.
- Thong-Asa W, Tilokskulchai K, Chompoopong S, Tantisira MH. Effect of Centella asiatica on pathophysiology of mild chronic cerebral hypoperfusion in rats. Avicenna J Phytomed. May-Jun 2018;8(3):210-226.
- Sahraei R, Aminyavari S, Hosseini M, Hassanzadeh-Taheri M, Foadoddini M, Saebipour MR. The Ameliorative Impact of Centella asiatica on the Working Memory Deficit in Streptozotocin-induced Rat Model of Alzheimer Disease. Basic Clin Neurosci. Jan-Feb 2022;13(1):25-34. doi:10.32598/bcn.2021.144.4
- Md Pisar M, Chee BJ, Long I, Osman A. Protective effects of Centella asiatica extract on spatial memory and learning deficits in animal model of systemic inflammation induced by lipopolysaccharide. Ann Med. Dec 2023;55(1):2224970. doi:10.1080/07853890.2023.2224970
- Awad R, Levac D, Cybulska P, Merali Z, Trudeau V, Arnason J. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Canadian journal of physiology and pharmacology. 2007;85(9):933-942.
- Sbrini G, Brivio P, Sangiovanni E, et al. Chronic Treatment with a Phytosomal Preparation Containing Centella asiatica L. and Curcuma longa L. Affects Local Protein Synthesis by Modulating the BDNF-mTOR-S6 Pathway. Biomedicines. Nov 26 2020;8(12)doi:10.3390/biomedicines8120544
- Jana U, Sur TK, Maity LN, Debnath PK, Bhattacharyya D. A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal Med Coll J. Mar 2010;12(1):8-11.
- Wattanathorn J, Mator L, Muchimapura S, et al. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J Ethnopharmacol. Mar 5 2008;116(2):325-32. doi:10.1016/j.jep.2007.11.038
- Ashour ML, Wink M. Genus Bupleurum: a review of its phytochemistry, pharmacology and modes of action. J Pharm Pharmacol. Mar 2011;63(3):305-21. doi:10.1111/j.2042-7158.2010.01170.x
- Li XQ, Song YN, Wang SJ, Rahman K, Zhu JY, Zhang H. Saikosaponins: a review of pharmacological effects. J Asian Nat Prod Res. May 2018;20(5):399-411. doi:10.1080/10286020.2018.1465937
- Park WH, Kang S, Piao Y, et al. Ethanol extract of Bupleurum falcatum and saikosaponins inhibit neuroinflammation via inhibition of NF-κB. J Ethnopharmacol. Nov 4 2015;174:37-44. doi:10.1016/j.jep.2015.07.039
- Lin X, Wu S, Wang Q, et al. Saikosaponin-D Reduces H(2)O(2)-Induced PC12 Cell Apoptosis by Removing ROS and Blocking MAPK-Dependent Oxidative Damage. Cell Mol Neurobiol. Nov 2016;36(8):1365-1375. doi:10.1007/s10571-016-0336-5
- Lee TH, Park S, You MH, Lim JH, Min SH, Kim BM. A potential therapeutic effect of saikosaponin C as a novel dual-target anti-Alzheimer agent. J Neurochem. Mar 2016;136(6):1232-1245. doi:10.1111/jnc.13515
- Wang X, Li S, Yu J, et al. Saikosaponin B2 ameliorates depression-induced microglia activation by inhibiting ferroptosis-mediated neuroinflammation and ER stress. J Ethnopharmacol. Nov 15 2023;316:116729. doi:10.1016/j.jep.2023.116729
- Giuffrè M, Merli N, Pugliatti M, Moretti R. The Metabolic Impact of Nonalcoholic Fatty Liver Disease on Cognitive Dysfunction: A Comprehensive Clinical and Pathophysiological Review. Int J Mol Sci. Mar 15 2024;25(6)doi:10.3390/ijms25063337
- Wang X, Feng Q, Xiao Y, Li P. Radix Bupleuri ameliorates depression by increasing nerve growth factor and brain-derived neurotrophic factor. Int J Clin Exp Med. 2015;8(6):9205-17.