Hormones Involved in Hunger and Satiety
The body has an internal system for obtaining nutrients required for survival. In a series of complex steps and pathways involving the digestive tract and central nervous system (CNS), the brain and gut communicate to signal hunger and direct the brain to prompt the body to seek out food.1
Upon consuming a certain amount of food, the body will signal satiety, the feeling of having enough food and no longer being hungry. These satiety signals shut off hunger and food-seeking cues. If the messengers in this system do not function correctly or the body does not respond appropriately, overeating can occur, contributing to weight gain and eventually obesity.
Hunger vs. Appetite
Hunger and appetite mean similar but different things. The terms “hunger” and “appetite” are often used interchangeably, but they are technically different. The instinctual feeling we know as hunger is caused by a physiological mechanism that signals a need to eat to maintain energy levels. Hunger signals are greatest before the start of a meal and decrease throughout the meal as satiation rises.1 If left unanswered, hunger can lead to physical symptoms including headache or discomfort.1
During the course of eating a meal, satiation will begin. Satiation is the process in the body that leads to the termination of eating.2 It is influenced by behavioral cues, biological mechanisms, and the context of the meal.2 Satiety, on the other hand, is the feeling of fullness that persists after eating, suppressing food intake between meals, until hunger returns.3 Both satiation and satiety can have a significant impact on food and caloric intake.
Appetite, on the other hand, is caused by the desire to eat. It can be a product of hunger, but it can also stem from external cues, including the environment, emotions, or social cues.4,5 Additionally, it may be satisfied by a specific, highly desirable food such as ice cream or potato chips, whereas hunger can be satisfied by any food that provides calories.
Hunger and Satiety Pathways
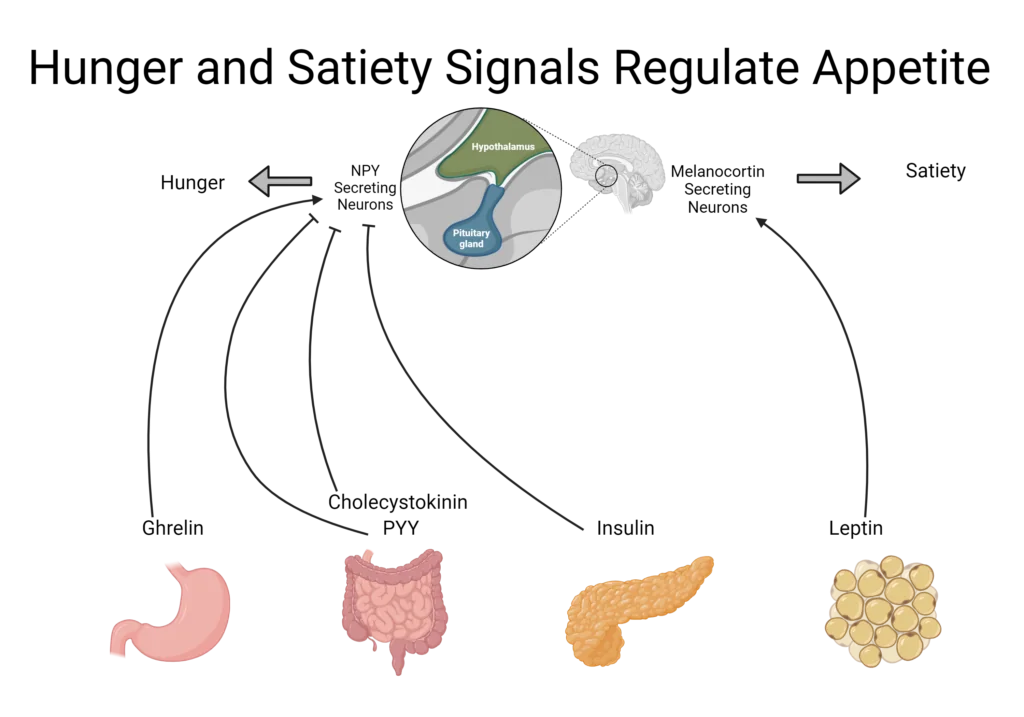
The gastrointestinal (GI) tract detects the presence or absence of food. When it notes an absence, hormonal signals generate hunger cues which exert their effects as chemical messengers on the GI tract and CNS.1 These messengers primarily target the hypothalamus which contains specific neurons involved in regulating digestion, hunger, and satiety.4
When food is present in the GI tract, satiety signals override hunger signals to promote the inhibition of food intake.1 Under homeostatic conditions hunger regulates energy intake to keep the body functioning properly and to keep body weight stable.4 When hunger cues become dysregulated, overeating can occur, leading to weight gain, metabolic issues, and ultimately obesity.1,4
Hunger and satiety rely on signals from the gut, brain, and peripheral tissues including white adipose tissue and the pancreas. These organs can sense the body’s status in real time, initiate the appropriate response, and terminate the signal when necessary. The cues and signals from the body are mostly in the form of peptide hormones, short chains of amino acids that function as hormones. They are also sometimes referred to as gut hormones because of their origin or function in the gut.
The mechanisms underlying hunger and satiety hormones are often similar, but they work in opposite directions. For example, hunger hormones may increase gut motility and gastric emptying to make room for more food whereas satiety hormones will slow these processes down, allowing time for the body to respond to the presence of food, feel satiated, and send satiety signals to the brain.1,4
Many of the hormones involved in inducing hunger or satiety also regulate other hormones and interact differentially with neurons at various centers in the brain and hypothalamus to exert their effects.4 Gut hormones involved in hunger and satiety can also regulate energy expenditure, the amount of energy a body uses to carry out essential processes. When caloric intake exceeds energy expenditure, weight gain occurs.4
Hormones Involved in Hunger and Satiety
Gut hormones are mainly categorized as orexigenic or anorexigenic. Orexigenic hormones stimulate hunger and appetite, working as critical signals to the body to prompt it to seek food and energy. Anorexigenic hormones signal satiety and suppress food intake often by inhibiting hunger cues. Together, they direct the body to increase or decrease food intake, maintaining the cycle of hunger and satiety.
Orexigenic Hormones
Ghrelin
Ghrelin, also known as the hunger hormone, induces hunger by stimulating gastric motility, accelerating gastric emptying, and increasing gastric acid secretion in anticipation of food before a meal.4,6,7 It also excites neurons involved in producing other appetite-stimulating hormones and inhibits satiety hormones.6
Ghrelin is secreted during a fasted state with levels peaking immediately before meal initiation then falling within one hour of consuming food, proportional to caloric and nutrition consumption.4,7 Fat is the least effective at lowering ghrelin while protein is the most effective.4 Sleep suppresses ghrelin, as part of the circadian rhythm independent of meals, whereas stress can increase ghrelin secretion.7
NPY
Neuropeptide Y (NPY) is produced in the GI tract as well as the hypothalamus where it induces hunger, promotes feeding, and reduces energy expenditure.6 Together, these can greatly impact caloric intake and contribute to weight gain.
Other orexigenic hormones include:4,6,7
- Orexin: Increases food intake and gastric motility
- Melanin concentrating hormone (MCH): Promotes hyperphagia, weight gain, and lipogenesis; involved in energy balance and emotional control
- Agouti-related protein (AgRP): Prevents anorectic effects of satiety hormones
Anorexigenic Hormones
Leptin
Leptin is produced in white adipose tissue, in proportion to the size of the adipocyte; however, it can also be released by from the stomach during a meal.4,6,8 This makes leptin both a short- and long-term satiety hormone.4,7 It targets the hypothalamus to regulate eating behavior as well as energy expenditure.6 Leptin suppresses appetite by mediating hunger hormones, decreasing food intake, and inducing satiety; it also increases energy expenditure.6-8
CCK
Cholecystokinin (CCK) is produced and functions in the small intestines and CNS.1,6 It is secreted in response to consumption of fat and protein, aiding in digestion by stimulating the production of bile and secretion of pancreatic enzymes, and delaying gastric emptying.,1,4,6,7 It also inhibits ghrelin and stimulates other satiety hormones.4,6
PYY
PYY (Peptide Tyrosine-Tyrosine) is produced in the small intestines but targets the CNS and hypothalamus.4,6,7 PYY is secreted proportionally to the quantity of food consumed but also in response to the macronutrient composition of the meal, with greater secretion following high protein meals compared to meals higher in carbohydrates and fat.4 It increases satiety, postponing consumption of the next meal, and delays gastric emptying.4,6
PP
PP (Pancreatic Polypeptide) is synthesized primarily in the pancreas with small quantities secreted from the large intestines.4,6,7 After food is ingested, PP is secreted in relation to the amount of calories ingested.4,6 Levels will peak within 15 minutes of eating, but they can remain elevated for up to six hours.7 PP slows gastric emptying and gut motility as well as contraction of the gallbladder to increase satiety.7 It can also modulate energy homeostasis.4,6
Additional hormones that suppress hunger include:4,6,7,9
- Amylin: Reduces food intake and promotes negative energy balance
- Corticotropin releasing hormone (CRH): Regulates energy balance
- Pro-opiomelanocortin (POMC): Signals satiety and reduces food intake; integrates signals from ghrelin, leptin, and insulin
- Oxyntomodulin (OXM): Reduces circulating ghrelin to decrease feelings of hunger, decreases gastric emptying, and inhibits gastric secretion
- Obestatin: Participates in gastric emptying and insulin release
- Resistin: Regulates energy homeostasis and affects insulin resistance
- Secretin: Activates brown adipose tissue to induce satiety
Glucose Metabolism-related Hormones
Several hormones involved in blood glucose metabolism are also important in inducing satiety. Because obesity is often accompanied by changes in glucose metabolism, hunger and satiety hormones can become very dysregulated when metabolic dysfunction occurs.4
Insulin and Related Hormones
Insulin is a peptide hormone secreted from the pancreas that signals the presence of energy and nutrients following a carbohydrate-containing meal.7 It modulates other satiety hormones, including increasing leptin production and decreasing NPY expression, helping to reduce food intake.6,8
Insulin can also cross the blood-brain barrier to bind to insulin receptors. In the brain, insulin influences several aspects of energy balance, metabolism, hunger, and neural activity.7 Insulin is both a short- and long-term regulator of satiety.7 Similarly, glucose-dependent insulinotropic polypeptide (GIP) stimulates the release of insulin and increases the sensation of fullness in the stomach, helping to decrease food intake.6
Glucagon and Related Hormones
Glucagon is considered a gut hormone due to its role in influencing food intake. While it exerts the opposite effect on blood glucose compared to insulin, it also reduces food intake.4 GLP-1, glucagon-like peptide 1, is synthesized in the small intestines and secreted in response to the presence of nutrients in the GI tract.7 GLP-1 receptors are found throughout the body including in the heart, kidney, muscles, and lungs.1,6 Its primary function is to decrease circulating glucose levels by increasing insulin levels and reducing glucagon, but it also signals satiation and slows gastric emptying to limit food intake.4,7
Other Hormones Involved in Hunger and Satiety
Scientists have successfully categorized many hormones as orexigenic or anorexigenic; however, the role of some hormones involved in hunger or satiety is more ambiguous. Some hormones exhibit complex effects and may have contradictory roles depending on factors such as nutritional state, dietary intake, and body mass. Two such hormones are somatostatin and adiponectin.
Somatostatin (SST) is widely distributed in the CNS as well as peripheral tissues including the pituitary gland, pancreas, and GI tract.10 It plays a significant role in GI tract physiology and functions as both an anorexigenic and orexigenic hormone, working with ghrelin to initiate food-seeking behaviors but also inducing satiety and regulating other satiety hormones.10
Adiponectin is primarily produced by adipose tissue, but its expression depends on multiple factors including age, sex, body weight, adipose tissue content, nutritional status, and dietary intake.6,11,12 It can cross the blood brain barrier where it binds to receptors in the CNS to regulate energy homeostasis, stimulate fatty acid oxidation, and improve insulin sensitivity.6 Studies have shown that adiponectin secretion results in decreased food intake while others have found it increases food intake or has no effect.10,11 It may also be able to modulate gastric motility, impacting satiety.13
Created with BioRender.com
Obesity-associated Changes in Satiety Hormones
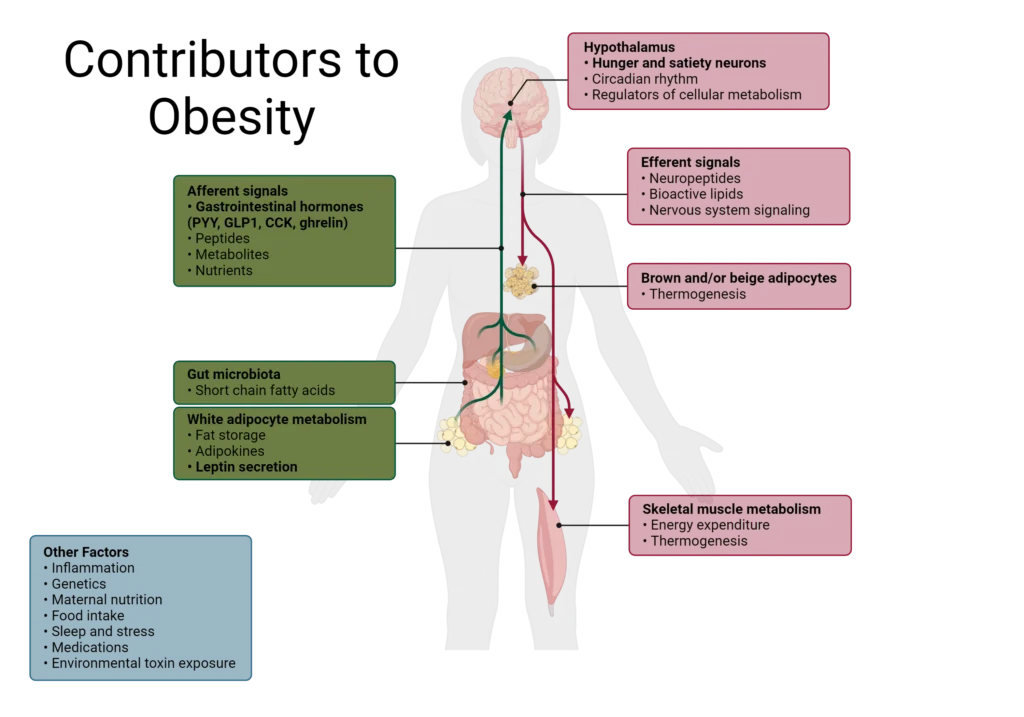
Overeating is a main cause of weight gain which can contribute to obesity over time.6 Gut hormones are often differentially regulated in individuals with obesity although it is not always clear if that is a cause or consequence of obesity.4 Oftentimes, obese individuals will demonstrate lower levels of satiety hormones and impaired suppression of hunger hormones.4,14
For example, GLP-1 response in an obese state are similar before a meal but attenuated in the postprandial response, resulting in a significantly blunted response.14 A similar effect was seen for PYY and corresponded to lower ratings of fullness in obese subjects.14 The relatively flattened hormone response in obesity may result in a continuous grazing pattern of eating, adding to daily calorie consumption and weight gain.14
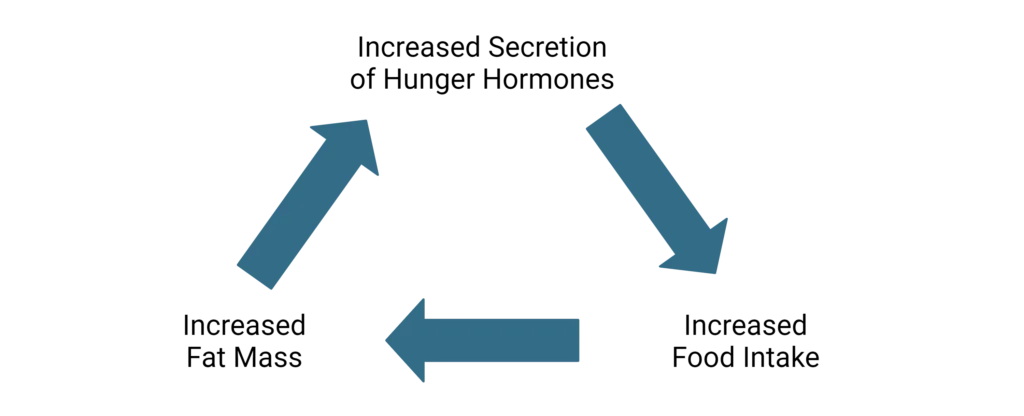
Changes in hormones that accompany obesity are not always easily predicted. For example, individuals with obesity often demonstrate increased levels of leptin before a meal, due to increased adipose tissue.14 However, leptin induces satiety which conflicts with reports of hunger in obese subjects. This led scientists to suggest the presence of central leptin resistance in obese individuals which disrupts normal satiety cues.14 For individuals who lose weight, specifically fat mass, leptin levels will decrease which will increase hunger and may ultimately contribute to gaining weight back.4
For most of human history, food intake was limited, so the body adapted to retain the most calories when it received food. Those mechanisms are poorly suited for the current obesogenic environment, where there is an abundance of calories and foods.4 The development of highly processed foods that can trigger an addiction-like response can also override the body’s natural satiety cues, leading to food consumption despite being full, resulting in weight gain and eventually development of obesity.4
Created with BioRender.com
The Role of Hunger and Satiety in Obesity and Metabolic Disorders
Appetite control evolved when food was relatively limited for most of history, meaning orexigenic signaling is strong and may not be suited to the modern environment of overnutrition.4 Additionally, the addictive nature of ultra-processed foods can override satiety signals, leading to overeating.4
Inflammation in the hypothalamus can also contribute to metabolic disturbances, including obesity, by altering the central regulation of appetite.4,15 As a result, conditions like leptin resistance may arise, in addition to insulin resistance and metabolic syndrome due to aberrant hormone and cellular signaling.
As obesity progresses, more components of energy homeostasis can become dysregulated, including the gut microbiome which controls some satiety signals as well as connects inflammation to the brain.15
Dysregulated hormone signaling, altered cellular metabolism, fat mass accumulation, and increased food consumption can quickly become a vicious cycle that is difficult to break. As such, early intervention or preventative strategies can greatly impact the development of hunger and satiety imbalance.
Breaking the cycle of overeating or intervening to reduce inflammation can help restore homeostasis along the gut-brain axis and throughout the body. Additionally, healthcare professionals can help individuals seeking additional information by establishing healthy eating habits and encouraging lifestyle changes to optimize hormonal balance and appetite regulation.
Created with BioRender.com
Nutritional Interventions for Managing Hunger and Satiety
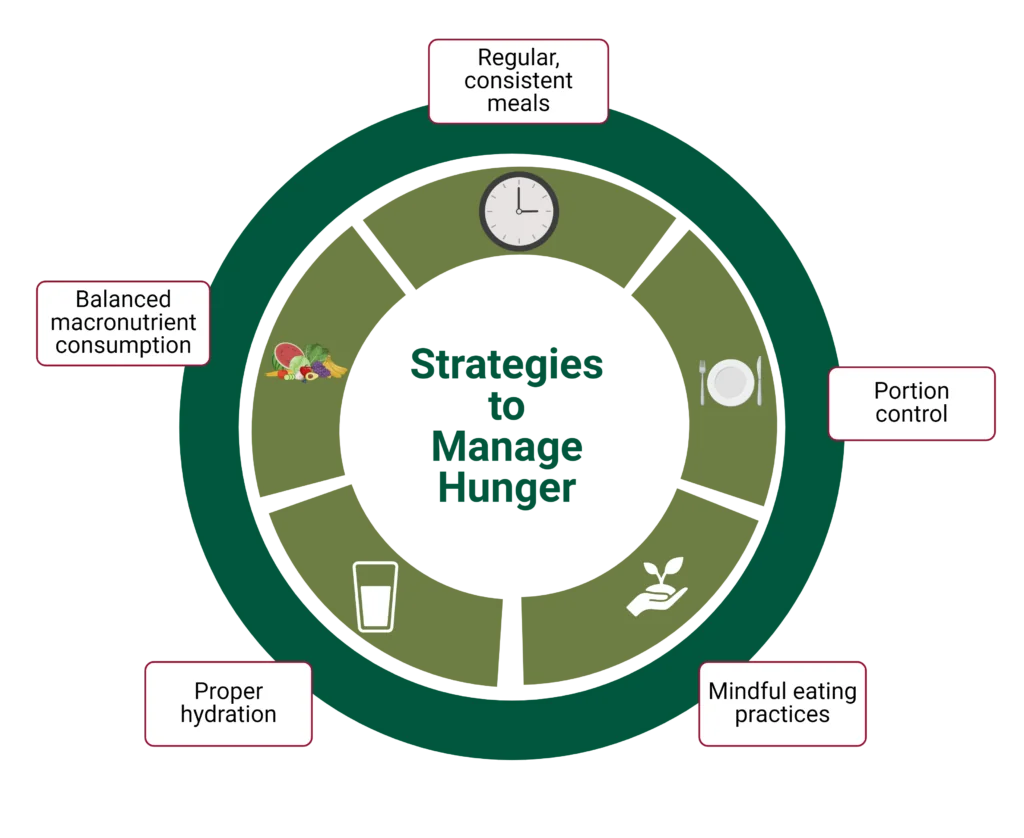
Hunger and satiety cues are maintained in a careful balance to control appetite regulation and disturbances that can lead to metabolic issues in the body. However, lifestyle modifications and practices can help manage hunger and optimize satiety cues, such as the following:
Balance Macronutrient Intake
Consume a blend of macronutrients at every meal to optimize the satiety response.
Complex carbohydrates, including fiber, delay gastric emptying, allow time for satiety cues to reach the brain, and inhibit food intake. Vegetables, beans, and lentils are good sources of fiber.
Whole food protein, such as from eggs, nuts, and lean meat, is the strongest inducer of the satiety signal released from the GI tract.
Consume Healthy Fats
Healthy fats including mono- and poly-unsaturated fatty acids promote satiety by delaying gastric emptying and support healthy inflammation throughout the body. Healthy fats can be found in avocados, walnuts, and salmon.
Focus on Meal Timing and Frequency
Consider meal timing and frequency to align with the body’s natural circadian rhythm and with the body’s natural hunger and satiety cues. Regularly timed meals can reduce the risk of overeating, stabilize blood sugar levels, and help maintain hunger hormones at a healthy level.
Become More Mindful of Eating
Practice mindful eating to enhance hunger and satiety awareness. It takes approximately 20 minutes for hormones from the gut to reach the brain, therefore eating slowly can help the brain recognize and respond to satiety cues, as well as help savor the food to promote great satisfaction and establish a healthy relationship with eating.
Be Strategic with Portion Control
Be strategic about portion sizes to prevent overeating. Small steps like using smaller plates, measuring portions, and practicing portion awareness through mindful eating can support appetite regulation.
Drink Plenty of Water
Stay hydrated and drink water before meals to promote feelings of fullness and reduce caloric intake. Mechanoreceptors are part of appetite regulation, so the ability of water to fill the stomach can leave less room for food as well as initiate the beginning of satiety cues. Staying hydrated throughout the day can also help the body function efficiently and may quench a feeling of hunger that was something else, such as being bored or thirsty.
Created with BioRender.com
WholisticMatters is powered by Standard Process, a nutritional supplement company that’s family-owned and has operated for over 90 years. Standard Process offers many nutritional and whole food-based solutions.
- Tack, J., Verbeure, W., Mori, H., Schol, J., Van den Houte, K., Huang, I.-H., Balsiger, L., Broeders, B., Colomier, E., Scarpellini, E., Carbone, F. (2021). The gastrointestinal tract in hunger and satiety signalling. United European Gastroenterol J, 9(6):727.
- Cunningham, P.M., Rolls, B.J. (2021). The Satiation Framework: Exploring processes that contribute to satiation. Physiol Behav, 236:113419.
- Benelam, B. (2009). Satiation, satiety and their effects on eating behaviour. Nutr Bull, 34:126.
- Alhabeeb, H., AlFaiz, A., Kutbi, E., AlShahrani, D., Alsuhail, A., AlRajhi, S., Alotaibi, N., Alotaibi, K., AlAmri, S., Alghamdi, S., AlJohani, N. (2021). Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients, 13:481.
- Howland, M., Hunger, J.M., Mann, T. (2012). Friends don’t let friends eat cookies: effects of restrictive eating norms on consumption among friends. Appetite, 59(2):505.
- Boix-Castejón, M., Roche, E., Olivares-Vicente, M., Álvarez-Martínez, F.J., Herranz-López, M., Micol, V. (2023). Plant compounds for obesity treatment through neuroendocrine regulation of hunger: A systematic review. Phytomedicine, 113:154735.
- Tacad, D.K.M., Tovar, A.P., Richardson, C.E., Horn, W.F., Krishnan, G.P., Keim, N.L., Krishnan, S. (2022). Satiety Associated with Calorie Restriction and Time-Restricted Feeding: Peripheral Hormones. Adv Nutr, 13:792.
- Borer, K.T. (2021). Why We Eat Too Much, Have an Easier Time Gaining Than Losing Weight, and Expend Too Little Energy: Suggestions for Counteracting or Mitigating These Problems. Nutrients, 13:3812.
- Sun, L., Laurila, S., Lahesmaa, M., Rebelos, E., Virtanen, K.A., Schnabl, K., Klingenspor, M., Nummenmaa, L., Nuutila, P. (2023). Secretin modulates appetite via brown adipose tissue-brain axis. Eur J Nucl Med Mol Imaging, 50(6):1597.
- Kumar, U., Singh S. (2020). Role of Somatostatin in the Regulation of Central and Peripheral Factors of Satiety and Obesity. Int J Mol Sci, 21:2568.
- Tang, N., Zhang, X., Chen, D., Li, Z. (2021). The Controversial Role of Adiponectin in Appetite Regulation of Animals. Nutrients, 13(10):3387.
- Janiszewska, J., Ostrowska, J., Szostak-Wę (2021). The Influence of Nutrition on Adiponectin- A Narrative Review. Nutrients, 13(5):1394.
- Idrizaj, E., Garella, R., Squecco, R., Baccari, M.C. (2020). Can adiponectin have an additional effect on the regulation of food intake by inducing gastric motor changes? World J Gastroenterol, 26(20):2472.
- Lean, M.E.J., Malkova, D. (2016). Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes (Lond), 40(4):622.
- Szalanczy, A.M., Key, C.-C.C., Woods, L.C.S. (2022). Genetic variation in satiety signaling and hypothalamic inflammation: merging fields for the study of obesity. J Nutr Biochem, 101:108928.







