The Human Gut Microbiome
What is the Gut Microbiome?
The gut microbiome is a vibrant microbial community located in the human gastrointestinal (GI) tract that is ultimately responsible for linking gut activity to many other functions in the body, such as the nervous, immune, and endocrine systems. Via the gut microbiome, mainly the lower GI tract, the human body plays host to nearly 39 trillion bacteria cells; there are about just as many bacterial cells in the body as there are human cells.1 Also known as gut microbiota, these bacteria can play multiple roles in human health, starting before birth and continuing throughout life.
The bacteria in the gut can be defined by their relationship with their human host. These types of bacteria and other organisms (viruses, fungi, etc.) make up the larger microbiome.
- Commensal bacteria do not elicit either beneficial or adverse effects on the host. Commensal bacteria may include Escherichia coli, streptococcus, and Bacteroides.
- Mutualistic bacteria, such as Lactobacillus spp, benefit from their host environment, and they repay that kindness with their own benefits to the host. Mutualistic bacteria produce short-chain fatty acids (SCFA) that further convert to butyrate. Butyrate can be stimulated by probiotics and prebiotics, which influence gut health, cardiovascular health, and other body systems.2
- Disruptive or pathogenic bacteria, such as Clostridium perfringens, harm the host.3
The “microbiome” includes the whole microbial habitat: all microorganisms, their genomes, and the conditions of the environment.4 The term “microbiota” refers to a group of microorganisms that focuses specifically on the effect on human health outcomes.5
Probiotics vs. Prebiotics
Busy schedules and stressful jobs often result in what has become a typical “Western diet” — abundant with convenient processed foods that can wreak havoc on the GI tract, resulting in fatigue, skin health issues, and digestive discomfort. Rather than shrugging off seemingly minor health issues, identifying root causes and proactively improving gut health can revitalize whole body health. Two important aspects of improving microbial diversity and overall health of the gut microbiome include probiotics and prebiotics.
- Probiotics consist of microorganisms that provide a health advantage to their human host when consumed. Probiotics are ingested either as a food (in yogurt or other fermented foods), through a supplement, or medical food.4
- Prebiotics are food components or supplements that cannot be digested by humans, such as fiber. Not all fibers are prebiotics, and not all prebiotics are fibers. Prebiotics have been described as a food source for the beneficial bacteria.4 Prebiotics provide a broader set of health benefits than fiber alone, including:
- improving infectious or antibiotic-associated diarrhea
- reducing inflammation associated with inflammatory bowel disease (IBD)
- protection from colon cancer
- improving bioavailability of minerals
- reducing risk of cardiovascular disease
- supporting weight management
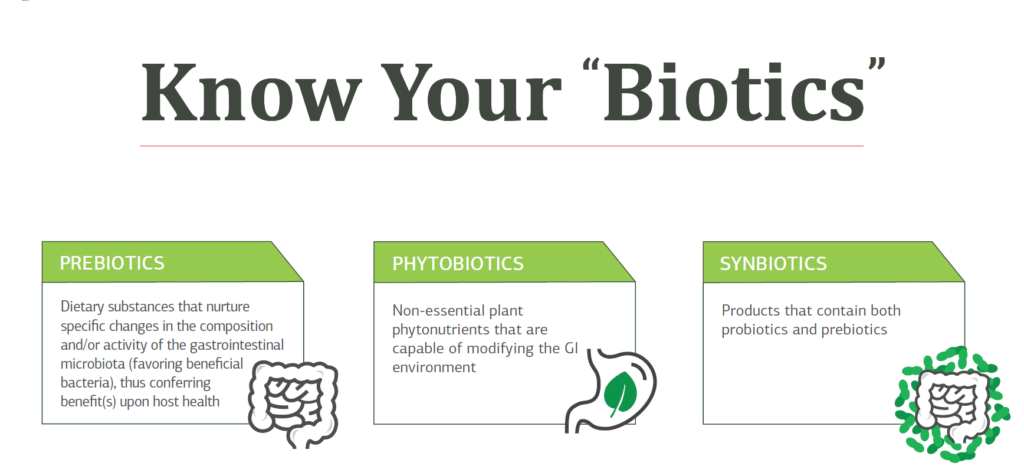
Additionally, synbiotics are a combination of both probiotics and prebiotics that feed a specific bacterial strain.
KNOW YOUR BIOTICS
Download the infographic!
Prebiotics, probiotics, synbiotics, phytobiotics, psychobiotics, parabiotics, and postbiotics explained.
The Metabolome of the Microbiome
The metabolome of the microbiome is a collection of small-molecule chemicals, including amino acids, enzymes, co-factors, and more produced by gut bacteria and genetically encoded to function in the body. The “food” or “gut” metabolome is influenced by what food is consumed and, particularly, the long-term dietary pattern followed. That means, even if a person eats a kale salad for lunch, it will not “overcome” a breakfast of donuts and dinner of pizza that represent the rest of their dietary pattern.
Appropriately, the term “metabolome” combines the words “metabolite” and “chromosome.” Over 25,000 compounds exist in foods consumed by humans, and the interaction of those compounds with the metabolome can produce dietary biomarkers that influence human health, though the mechanisms are not yet fully understood.6
Researchers have identified the metabolome of the typical Western dietary pattern through principal component analysis (PCA). Each dietary pattern has its own metabolic “signature” that is characterized by levels of amino acids and acylcarnitines. Foods typical of the Western diet have increased levels of branched-chain amino acids and short-chain acylcarnitines.7 Other research has found that higher levels of short-chain acylcarnitines are associated with higher risk of cardiovascular disease and stroke. Conversely, individuals who follow Mediterranean dietary patterns, characterized by high intake of fruits, vegetables, whole grains, legumes, fish, and poultry, have lower risk of cardiovascular disease, along with lower levels of short-chain acylcarnitines.8
While many gut health interventions focus on probiotic supplements, research indicates that gut health is much more complex. As a “healthy” microbiome is characterized, though, it will be possible to tailor dietary interventions both to improve the diversity of the microbiome and the metabolome of the microbiome. For example, in the case of obesity, researchers found that individuals who had a less diverse microbiome were more likely to have dysbiosis in the gut and higher levels of systemic inflammation.9
Plant-based dietary patterns like the Mediterranean diet appear to have a beneficial effect on gut health, including the enhanced diversity of microbial species in the gut and the resulting metabolome of the microbiome. The foods humans eat impact health beyond the macronutrients and micronutrients in the food and their influence on the body. Perhaps the “second brain” of the gut microbiome should be considered a “second metabolism” — influencing the development of conditions like hypertension, dyslipidemia, and cardiovascular disease. In a clinical setting, it may soon be possible to identify individuals who are more susceptible to chronic inflammatory conditions based on their metabolic and microbiome profiles.
Lifestyle Influences on the Microbiome
The health of the gut microbiome is a foundational part of digestive health. Scientific evidence makes significant connections between microbiome diversity, diet, and physiological effects – good and bad.17 For example, poor microbiome health is linked to increased levels of inflammatory cytokines.18
The human microbiome begins to develop in infancy. After birth and during early maturation, exposure to new energy sources from food and the external environment shapes the content of the microbiome until a young child’s microbiome reaches “adult status.”19 Many factors may affect the content and diversity of the human microbiome: antibiotic use, prolonged prescription drug use, dietary changes, gastrointestinal illness, development of chronic conditions, moving to a new country, short term travel, and even the stressful holiday season.
Antibiotics and GI Health
Antibiotics can be detrimental to GI health by negatively influencing the growth of helpful microbiota, lowering biodiversity and disrupting the production of metabolic byproducts from phytonutrients, compromising intestinal barrier integrity (possibly leading to leaky gut), and favoring the growth of opportunistic pathogens. Antibiotic-related GI damage can be especially common in the elderly, as they are one of the most commonly prescribed medications for this age group.20 Additional prescription medications, such as proton pump inhibitors and antipsychotics result in lower diversity of the microbiome.
The Role of Microbiota During Exercise-induced Stress
The gut and the microbiota have important functions during exercise because they are responsible for the delivery of water, nutrients, hormones, and neurotransmitters, while also contributing to enteric immunity and the regulation of inflammation and oxidative stress.18 The main microorganisms that respond to exercise are Firmicutes and Actinobacteria, which contain the Lactobacillus and Bifidobacterium genera. Some of these will produce SCFA in response to exercise.19
In the lower intestine, polysaccharides from the diet are digested and subsequently fermented by Lactobacillus and Bifidobacterium into beneficial SCFAs which are used as energy sources.20 The microbiota-produced SCFAs affect a range of host processes including colonic pH, microbiota composition, intestinal motility, gut permeability, and epithelial cell proliferation.21 Studies have shown that exercise can not only affect SCFA production in the intestine, but can in turn affect the HPA axis, the health of the GI tract, and may potentially promote physical performance. A 2016 systematic review on endurance exercise suggested that gut microbiota have a key role in controlling oxidative stress and inflammatory responses, and it can also help improve body metabolism and energy expenditure during exercise.22
Diet, Probiotics, and the Microbiota
Dietary recommendations to optimize performance during exercise are well-known, but rarely is the health of the gut microbiota considered. In general, recommendations for exercise often encourage consumption of high amounts of simple carbohydrates and protein and low amounts of fat and fiber in order to provide a quick source of energy while attempting to avoid potential gas and distension, which high fiber diets can sometimes cause. However, insufficient consumption of fiber may promote a loss of microbial diversity in the GI tract.23 Growing research suggests that this may play a role in the development and function of an appropriate stress response.
Therefore, dietary and supplement recommendations aimed to reduce symptoms of exercise-induced stress by improving gut microbiota composition during exercise are of growing interest. For example, ensuring adequate intake of dietary fiber, plant-based foods, and synbiotics (a combination of probiotics and prebiotics that feed specific bacterial strains), helps to ensure one’s ability to feed commensal bacteria that produce beneficial by-products for host metabolism and homeostasis (e.g. SCFA and neurotransmitters), and inhibit bacterial production of potentially harmful metabolites.24 As the diet strongly influences microbiota composition and function, modulation of the gut microbiota via nutritional and herbal modifications may improve the stress response and physical performance.
- Carbohydrates: Diets high in simple and refined carbohydrates do not promote a healthy gut microbiota composition nor do they produce beneficial SCFA.25
- Protein: Increased requirements during exercise are associated with a fall in the plasma concentration of glutamine, and it has been hypothesized that this decrease could impair immune function and increase susceptibility to infection and leaky gut.26
- Fats and polyunsaturated fatty acids: Omega-3 polyunsaturated fatty acids may decrease the production of inflammatory eicosanoids, cytokines, and reactive oxygen species (ROS) during exercise.27
- Vitamins and antioxidants: Vitamins C and E, β-carotene, and polyphenols may help reduce ROS formation and lipid peroxidation during exercise.28
- Probiotics: Can help promote healthy digestive function, reduce inflammation and intestinal permeability, and be of particular importance in those who experience GI symptoms in relation to exercise and stress by positively modifying the gut microbiota.29.30 Currently, Bifidobacterium strains have generated the best results.31
- Herbal Adaptogens: Plants such as Korean ginseng (Panax ginseng), Schisandra berry (Schisandra chinensis), Rhodiola rosea, and Ashwagandha (Withania somnifera) increase the body’s resistance to physical and emotional stress and can strengthen compromised organ systems and body functions in the face of various stressors.32
Microbiome Across the Lifespan
The microbiome adjusts and shifts throughout the lifespan due to diet, medications (antibiotics), exercise, environment, disease states, and more. Supporting the microbiome with a diet that includes prebiotics and probiotics may have a significant impact through every stage of life.
Birth and Infancy
The method of delivery of an infant (vaginal versus caesarean delivery) has a significant influence on the make-up of the microbiota.10 Bifidobacteria is one of the predominate bacterial species in infants who are born via vaginal delivery. A baby’s microbiota is similar to the microbiota of the vagina of the mother. Alternatively, those infants delivered by caesarean are likely to have microbiota that are similar to that of the mother’s skin, which consists of high levels of Escherichia coli, Clostridium difficile, Bacteroides fragilis, and lactobacilli.
Infants who are fed manufactured formula milk have a different microbiota composition than do those who are fed breast milk. However, by providing prebiotics like galacto-oligosaccharides (GOS), inulin, and fructo-oligosaccharides (FOS) to formula-fed children, the microbiota can shift to increase Bifidobacteria population.11 Infant formulas with GOS and FOS have been available for years, but formula companies also have recently been able to add human milk oligosaccharides (HMOs) into many commercially-available infant formulas. HMOs like 2’-Fucosyllactose (2’-FL) are unique prebiotics that primarily feed Bifidobacteria, which are some of the first microorganisms to colonize the GI tract. Like other beneficial microbes, Bifidobacteria are associated with various health benefits such as addressing GI disorders, intestinal regularity, and reducing the risk of colon cancer.13-16
The benefits of breastfeeding are vast, including bonding of mother and baby, providing essential nutrients for growing babies, and providing important HMOs, but many infants are fed formula in addition to or instead of breastmilk. For those children, the gut microbiome can now be optimized with new formula options.
Aging
As humans age, the microbiome changes. Changes in the microbiome associated with age may result in an imbalance of Firmicutes to Bacteroidetes, causing dysbiosis.11 A recent study showed that as adults age, there is a reduction in total bacteria, which lowers the level of butyrate-producing bacteria (Firmicutes). These changes in the microbiome may account for the rising incidence of Clostridium difficile in nursing facilities.12
-
- Abbott, A. (2016). Scientists bust myth that our bodies have more bacteria than human cells. Nature. doi: 10.1038/nature.2016.19136
- Wong, J. M., Souza, R. D., Kendall, C. W., Emam, A., & Jenkins, D. J. (2006). Colonic health: fermentation and short chain fatty acids. Journal of Clinical Gastroenterology, 40(3), 2.
- Sekirov, I., Russell, S. L., Antunes, L. C., & Finlay, B. B. (2010). Gut microbiota in health and disease. Physiological Reviews, 90(3), 859-904. doi:10.1152/physrev.00045.2009
- Marchesi, J. R., & Ravel, J. (2015). The vocabulary of microbiome research: a proposal. Microbiome, 3(1). doi:10.1186/s40168-015-0094-5
- Lederberg, J., McCray, AT. (2001). ‘Ome sweet ‘omics – a genealogical treasury of words. Scientist.15(7):8–8.
- Scalbert, A., Brennan, L., Manach, C., Andres-Lacueva, C., Dragsted, L. O., Draper, J., Rappaport, S. M., van der Hooft, J. J., & Wishart, D. S. (2014). The food metabolome: a window over dietary exposure. The American journal of clinical nutrition, 99(6), 1286–1308. https://doi.org/10.3945/ajcn.113.076133
- Bouchard-Mercier, A., Rudkowska, I., Lemieux, S. et al.(2013). The metabolic signature associated with the Western dietary pattern: a cross-sectional study. Nutr J 12, https://doi.org/10.1186/1475-2891-12-158
- Guasch-Ferré M, Zheng Y, et al. (2016, June). Plasma acylcarnitines and risk of cardiovascular disease: effect of Mediterranean diet interventions. Am J Clin Nutr, 103(6):1408-16.
- Cotillard, A., Kennedy, S. P., Kong, L. C., Prifti, E., Pons, N., Le Chatelier, E., Almeida, M., Quinquis, B., Levenez, F., Galleron, N., Gougis, S., Rizkalla, S., Batto, J. M., Renault, P., ANR MicroObes consortium, Doré, J., Zucker, J. D., Clément, K., & Ehrlich, S. D. (2013). Dietary intervention impact on gut microbial gene richness. Nature, 500(7464), 585–588. https://doi.org/10.1038/nature12480
- Biasucci, G., Benenati, B., Morelli, L., Bessi, E., and Boehm, G. (2008). Cesarean delivery may affect the early biodiversity of intestinal bacteria. Nutr. 138, 1796S–1800S.
- Ottman, N., Smidt, H., Vos, W. M., & Belzer, C. (2012). The function of our microbiota: who is out there and what do they do? Frontiers in Cellular and Infection Microbiology, 2. doi:10.3389/fcimb.2012.00104
- Hunter, JC., Mu, Y., Dumyati, GK., et al. (2016). Burden of nursing home-onset Clostridium difficile infection in the United States: estimates of incidence and patient outcomes. Open Forum Infectious Diseases; 3(1):ofv196. doi:10.1093/ofid/ofv196.
- Roberfroid, M., et al. (2010). Prebiotic effects: metabolic and health benefits. Br J Nutr, 104 (2), S1-63.
- Williams, E.A., et al. (2009). Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther, 29(1), 97-103.
- Whorwell, P.J., et al. (2006). Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol, 101(7), 1581-90.
- Beachey, E.H. (1981). Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis, 143(3), 325-45.
- Dong, T.S. & Gupta, A. (2019). Influence of early life, diet, and the environment on the microbiome. Clin Gastroenterol Hepatol, 17(2), 231-242.
- Grenham, S., et al. (2011). Brain-gut-microbe communication in health and disease. Front Physiol, 2, 94.
- Lozupone, C.A., et al. (2012). Diversity, stability and resilience of the human gut microbiota. Nature, 489(7415), 220.
- (2020, June 11). Core elements of antibiotic stewardship for nursing homes. Retrieved from https://www.cdc.gov/longtermcare/prevention/antibiotic-stewardship.html







