Natural Ways to Support Methylation: Lifestyle Adjustments for Optimal Health
If you’re interested in health and wellness, chances are, you’ve heard of the term “methylation”.
What is Methylation?
Methylation is a fundamental biochemical process that involves the addition of a chemical group, known as a methyl group (-CH3), to our DNA, determining which genes are active and which are silenced. In this way, methylation acts like a genetic “on/off switch”, using information from our environment, diet, and lifestyle to influence how a gene expresses itself.
Understanding this concept of methylation may help to clarify the complex mechanisms through which our dietary habits, lifestyle choices, and environment may contribute to disease risk through a process known as epigenetics.
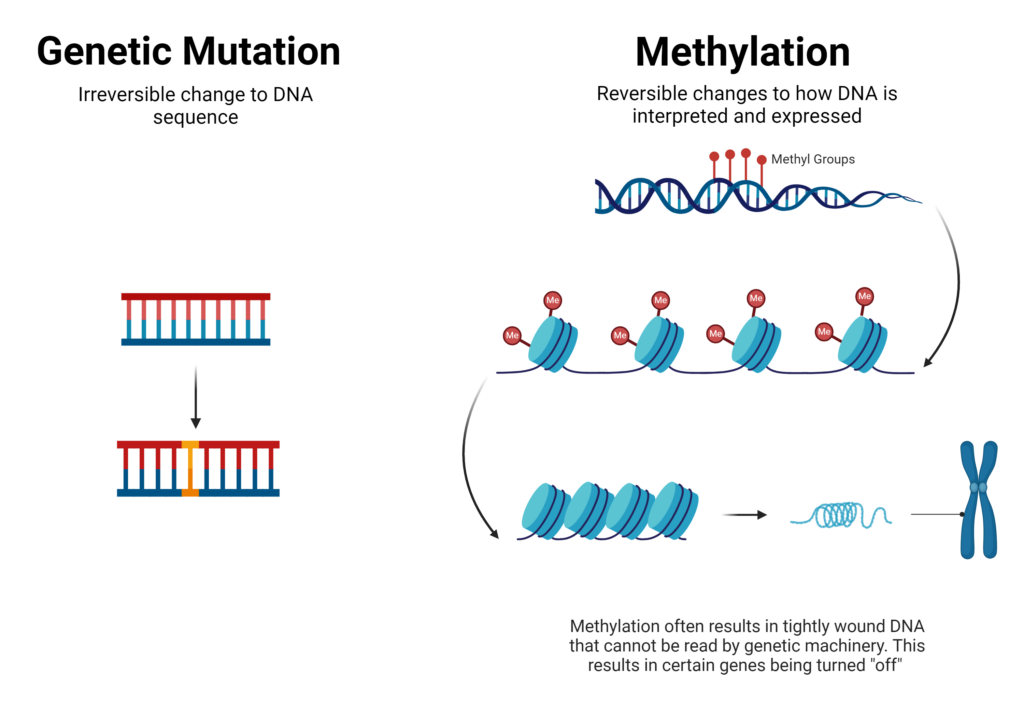
The Difference Between Methylation and Genetic Mutations
Because methylation changes the expression of our genes and not the actual DNA sequence, it is entirely different than genetic mutations. Genetic mutations are irreversible changes to the actual sequence of DNA, which can be inherited (i.e. passed down through generations) or acquired.
You can think of this in a similar way to a code for computers. If the “normal” code is ABCDEF, a genetic mutation would be described as a change in this code, like ABDDEF. Now, you receive two copies of every gene – one copy from your mother and the other from your father.
In inherited mutations, a mutated gene may be passed down by your mother, your father, or both. Unlike inherited mutations, acquired mutations are not passed down from a parent/parents, but rather occur in a person throughout their lifetime.
The Concept of Epigenetics
Epigenetics, on the other hand, means above genetics, or “that which controls the organism beyond genetics”.1 This in mind, epigenetic changes like methylation are reversible as they change how our body interprets and expresses our DNA, rather than changing the actual sequence.
To put it simply, you can think of our DNA as computer hardware and epigenetics as software. The computer equipment, much like the sequence of our DNA, doesn’t change, but we can change the software whenever we’d like.
We can download new apps to change the function of our computer, and thanks to epigenetics, the way that our DNA expresses itself can also change in response to our environment, experiences, lifestyle, and diet. These epigenetic or software changes determine which genes are turned on or off, ultimately influencing our body’s production of proteins that are involved in various processes like detoxification, neurotransmitter production, and cellular repair.
The Biochemical Process of Methylation
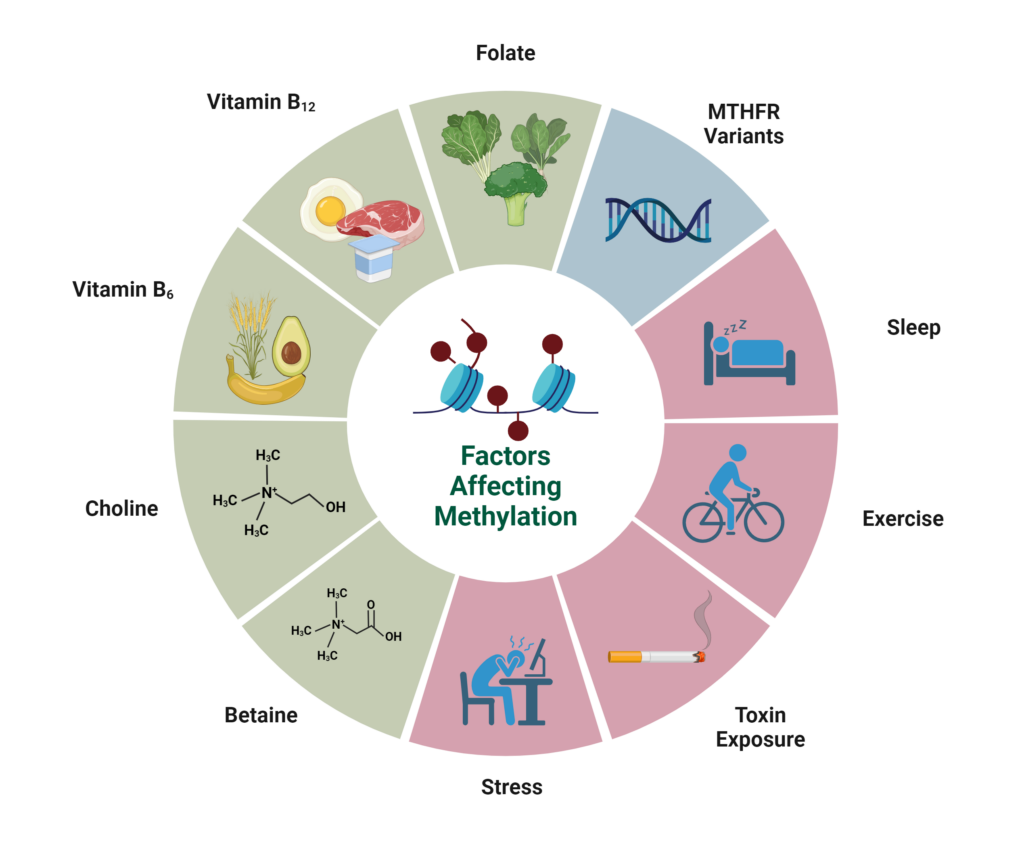
As a biochemical process, methylation is greatly dependent on the presence of certain nutrients that act as co-factors and methyl donors to keep the process moving smoothly. Nutrients like folate, choline, betaine, methionine, and vitamin B12, – serve as methyl donors, giving away methyl groups that can be used to turn genes on or off. Vitamin B6 is peripherally involved in this process, serving as a cofactor for alternate pathways.
Perhaps the most important nutrients to support methylation are folate and vitamin B12, as the body uses these nutrients to create the major methyl donor, SAMe . This process is known as the methionine cycle.2 The end goal of the methionine cycle is to continuously produce methyl donors that – power the methylation process. In the diet, folate and B12 are present in various forms, but the active forms are 5-methyltetrahydrofolate (5-MTHF), and methylcobalamin, respectively.
Let’s break down the methionine cycle a bit further.
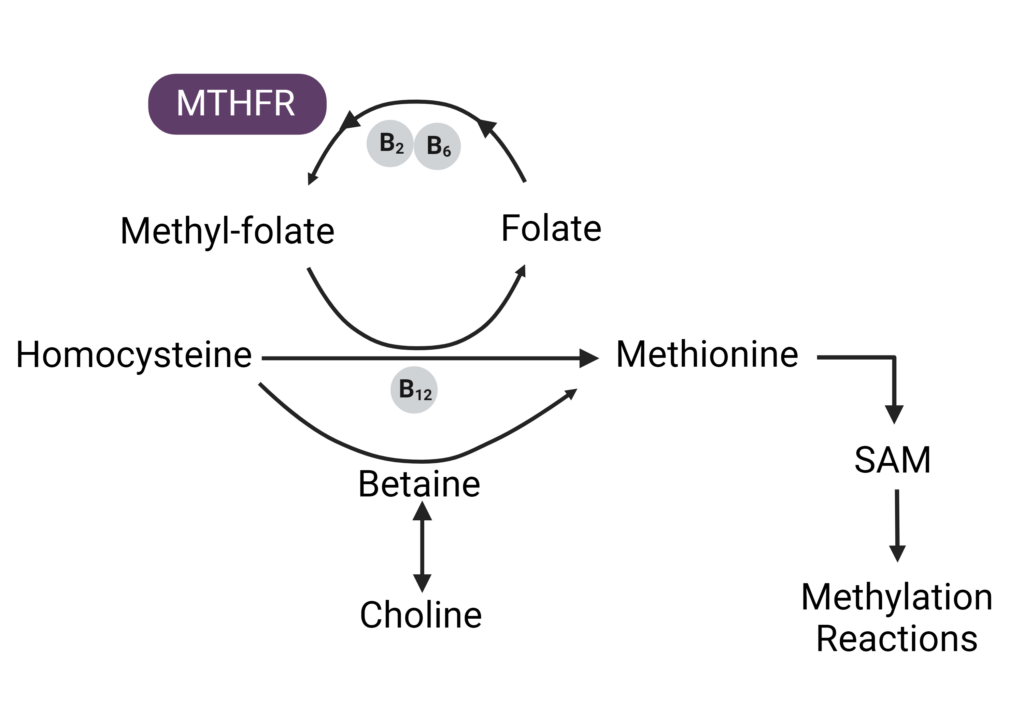
The Importance of Folate and Vitamin B12 in Methylation
Vitamin B12 and folate work together to transform homocysteine into methionine. The active form of vitamin B12 (methylcobalamin) transfers a methyl group to homocysteine with the help of the enzyme methionine synthase, transforming homocysteine into methionine. Folate then donates its methyl group to B12 to reactivate it for another round.
– Methionine is ultimately converted into a compound known as S-Adenosylmethionine, or SAMe, the body’s primary methyl donor. When SAMe donates a methyl group it comes S-Adenosylhomocysteine (SAH), which breaks down into homocysteine again, restarting the whole cycle.
If there is a deficiency in folate or B12, it can throw a wrench into this entire process-allowing homocysteine levels to build up. Homocysteine can be converted into cysteine with vitamin B6 as a cofactor, but elevated levels of SAH can interfere with this process.
Elevated levels of homocysteine can increase the risk of atherosclerosis by causing endothelial injury, promoting inflammation, and increasing oxidative stress. This phenomenon, known as hyperhomocysteinemia, is an independent risk factor for cardiovascular, cerebrovascular, and thromboembolic diseases thanks to the vascular inflammation it promotes.3
Understanding MTHFR Variants and Their Impact
While we are on the topic of MTHFR, it is important to address variants, otherwise known as polymorphisms, that may be present, as they can impact both folate and vitamin B12 status, thus predisposing to hyperhomocysteinemia as well.
A polymorphism is a variant within a gene that does not necessarily affect its function, unlike a pathogenic genetic mutation.4
Polymorphisms in the MTHFR Gene
One of the most common examples of polymorphisms is eye color; different variations in the DNA sequence of the eye color gene determine the amount of pigment produced in the eye, resulting in different eye colors. With this in mind, variations or polymorphisms in the MTHFR gene can influence the amount of active folate (5-MTHF) being produced from synthetic folic acid via methylation, but not all variations will cause detrimental effects.
Two predominant MTHFR variants occur within this gene: 677C>T and 1298A>C. Simply put, this means that at the 677th “letter” in the gene, the expected DNA base “C” is replaced by a “T.” For 1298A>C, this means that at the 1298th “letter” of the gene, the expected “A” base is replaced by a “C”.
Since these variants occur at two different places in the same gene, individuals can have variations in both “letters” at the same time, or they can have variations in only one and not the other. It’s also important to remember that you have two copies of the MTHFR “code”, as one comes from your mother, and one comes from your father. This means you can have one “normal” copy of the 677 letter from one parent and a variation of the 677 letter from the other parent. The same applies to the 1298 letter position.
In the general population, 60–70% of individuals will have at least one of these variants. 8.5% will have two copies of the 677C>T or the 1298A>C variation, meaning both the mother and father passed down the same variation at either the 677 or 1298 letter.
In 2.25% of the population, individuals will have one normal copy and one variated copy of both.4 The only cases in which these variations result in an actual change in the activity of the MTHFR enzyme are if individuals have two copies of the 677 variant, passed down from both mother and father or if an individual has one or two copies of the 1298 variant paired with a variation at the 677 “letter”. If an individual only has variations at the 1298 letter, there is no major change in enzyme activity.
Dietary Sources of Key Methyl Donors
So, why do these variations matter? If an individual falls into one of the above populations where the variations in the MTHFR gene impacts its activity, not only does this influence the body’s ability to convert folate to its active form but its ability to convert vitamin B12 into its active form as well because active folate re-methylates vitamin B12 after B12 donates its methyl group to homocysteine. These variants can predispose individuals to hyperhomocysteinemia as their bodies are not producing the metabolically active forms as efficiently, reducing available methyl donors.
Because vitamins play such a crucial role in the biochemistry of homocysteine, adequate dietary intake of nutrients like folate, vitamin B12, and vitamin B6 are key to ensuring healthy homocysteine levels.
That said, let’s review the dietary sources of these nutrients.
Folate
Folate is naturally present in a wide variety of foods, including vegetables (especially dark green leafy vegetables), fruits, nuts, beans, peas, seafood, eggs, dairy products, meat, poultry, and grains. Spinach, liver, asparagus, and brussels sprouts are among the foods with the highest folate levels.
Given the importance of folate, the FDA began requiring manufacturers to – fortify bread, cereals, flour, corn meals, pasta, rice, and other grain products with folic acid, a synthesized form of folate, in 1998.5
Folate Supplementation
Folate can also be taken in the form of supplements if needed, either in folic acid or metabolically active methyl-folate forms. Individuals with certain MTHFR variants may be recommended to supplement with the metabolically active methyl-folate form, given that the processes that complete this conversion in the body may be slowed.
Vitamin B12
Vitamin B12 is present in foods of animal origin, including fish, meat, poultry, eggs, and dairy products. It’s important to note that plant foods do not naturally contain vitamin B12, however certain algae may be a source, and many breakfast cereals and nutritional yeasts are fortified with bioavailable forms of this nutrient. 6
Vitamin B12 Supplementation
If dietary intake is not adequate, you can ask your healthcare practitioner about supplementation with vitamin B12 (i.e. cyanocobalamin, methylcobalamin, adenosylcobalamin, hydroxocobalamin).
Individuals with certain MTHFR variants may be recommended to supplement with the metabolically active form of vitamin B12, methylcobalamin, as the pathways that convert other forms of B12 (i.e. cyanocobalamin, hydroxocobalamin) may be slowed. However, these individuals can still convert cyanocobalamin and hydroxocobalamin into their methylated form, just at a decreased rate.
Vitamin B6
The richest sources of vitamin B6 include fish, beef liver and other organ meats, potatoes and other starchy vegetables, fortified cereals, and fruits like bananas.
Vitamin B6 can also be taken in supplemental form, so if you feel your dietary intake is lacking, you can talk to your healthcare provider to determine what is best for you.
The above B vitamins, while crucial to the homocysteine cycle, are not the only nutrients important for methylation.
Additional Nutrients Supporting Methylation
As mentioned above, betaine and choline are two additional nutrients that serve as methyl donors and can be used as backups if folate levels are not sufficient.
Choline is an essential nutrient that plays roles in addition to methylation, like forming the membranes of our cells.7 Choline can be converted to betaine, which then serves as a methyl donor. When we are deprived of choline, our bodies will rely more heavily on folate to recycle homocysteine, increasing our dietary folate requirements.8
In folate-deficient states, the body may heavily depend on betaine as a source of methyl groups.9 With this relationship in mind, it is also vital to ensure adequate intake of choline and betaine to support our methylation processes.
Let’s review food sources of these nutrients.
Choline
Although most Americans do not meet their body’s needs for choline, many foods contain this nutrient like meats, eggs, fish, dairy, potatoes, and cruciferous vegetables such as brussels sprouts, broccoli, and cauliflower.7
Some types of beans, nuts, and seeds also contain choline. Choline can also be taken in supplemental form to augment dietary intake.
Betaine
The most common dietary sources of betaine are beets, spinach, and whole grains like quinoa, wheat, oat brans, and brown rice.10
Lifestyle Factors Influencing Methylation
In addition to diet, lifestyle factors can also influence methylation capacity.
Stress
Chronic stress has been shown in research to deplete methyl donor nutrients, such as vitamin B6 and vitamin B12.11-12
Many neurotransmitters produced during times of prolonged stress, like adrenaline, require vitamin B6 and vitamin B12 for their production.13-14
As a result, long-term production of these neurotransmitters can increase vitamin B6 and B12 needs, and thus risk for deficiency. In addition to the depletion of these nutrients, chronic stress can also negatively change DNA methylation patterns.
For example, childhood abuse has been shown to increase methylation of the serotonin transporter gene SLC6A4 resulting in lowered expression of this transporter.15 This gene is known to regulate the availability of serotonin, enabling it to moderate aspects of emotional behavior.
With this in mind, heightened methylation of this gene resulted in lowered serotonin availability and thus lower quality of life, lower social and occupational functioning, and a higher level of disability.
Stress management practices like meditation and exercise should be considered to combat chronic stress and decrease the possibility of methylation disruption.
Toxins
It’s widely known that exposure to toxins in our environment and diet can have negative impacts on health and these effects can occur on the DNA level. Exposure to BPA, a chemical widely used in plastic production, can increase cancer cell proliferation through changes to DNA methylation.
Exposure to BPA was found to increase methylation of the TET2 gene, a tumor suppressor gene that functions to do just that – suppress tumor development.16
By increasing its methylation, exposure to BPA inhibits the expression of this beneficial gene, increasing the proliferation of ER-positive breast cancer cells. To minimize toxin exposure and its deleterious effects on methylation, reduce exposure to pollutants and opt for organic food when possible.
Exercise
Physical activity has a wide array of benefits for human health including DNA methylation. A study found that a combination of aerobic exercise and strength training increased the methylation of the RANKL gene, which promotes the breakdown of bone.17
By increasing the methylation of this gene, it becomes silenced, reducing bone breakdown and vulnerability to osteoporosis. Incorporate regular aerobic and strength exercises into your routine to gain the molecular benefits of healthy methylation.
Sleep
Sleep is yet another lifestyle factor that has significant effects on methylation capacity and overall health. It is well known that sleep deprivation causes negative cognitive changes, and recent research has unveiled one related molecular mechanism.
Studies have shown that sleep deprivation increases methylation of the FOXP2 gene which is involved in motor planning & learning.18
Hypermethylation and the resultant decreased expression of this gene results in language impairment.19 With this in mind, maintaining a regular sleep schedule and creating a restful sleep environment are key factors in maintaining healthy DNA methylation patterns.
Taking Control of Methylation through Diet and Lifestyle
In all, methylation is a vital yet extremely complicated biochemical process that underlies all aspects of health and wellness. While we may not have the capacity to change our genetic makeup which is set in stone from birth, this process highlights the importance of diet, lifestyle, and our environment in terms of our health.
Although research is still new and ongoing in the world of epigenetics and methylation, we have the power to take steps now, to enhance the expression of our genes for the better.
Ensuring adequate intake of methyl donor nutrients through diet and supplementation, minimizing toxin exposure, and adopting healthy lifestyle habits like regular exercise and sleep can ensure our genes are working with us, and not against us.
- National Institutes of Health. (n.d.). Genetics, epigenetics, and pregenetics. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK554408/
- Fuchs, J. E., et al. (2022). Nutriepigenetic regulation by folate–homocysteine–methionine axis: A review. Molecular and Cellular Biochemistry, [Springer]. https://link.springer.com/article/10.1007/s11010-013-1869-2
- McCully, K. S., & Jacobsen, D. W. (2022). Hyperhomocysteinemia. In StatPearls. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK554408/
- The Royal Australian College of General Practitioners (RACGP). (n.d.). MTHFR genetic testing: Controversy and clinical implications. Retrieved from https://www.racgp.org.au/afp/2016/april/mthfr-genetic-testing-controversy-and-clinical-imp
- National Institutes of Health. (n.d.). Folate – Health professional fact sheet. Retrieved from https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/#h3
- National Institutes of Health. (n.d.). Vitamin B12 – Health professional fact sheet. Retrieved from https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/#:~:text=Vitamin%20B12%20is%20present%20in,bioavailability%20%5B13%2C14%5D.
- National Institutes of Health. (n.d.). Choline – Consumer fact sheet. Retrieved from https://ods.od.nih.gov/factsheets/Choline-Consumer/
- Choi, S. W., & Mason, J. B. (2000). Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine, and choline. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 462(2-3), 77–84. https://www.sciencedirect.com/science/article/pii/S0022316622153794
- Sunden, S. L., et al. (2017). The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Journal of Molecular Medicine, 95, 15–21. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3798916/#:~:text=In%20folate%2Ddeficient%20patients%2C%20plasma,methionine%20and%20DMG%20%5B51%5D
- Zeisel, S. H., et al. (2020). Betaine as a functional ingredient: Metabolism, health-promoting attributes, food sources, applications and analysis methods. Journal of Nutrition, 150(Supplement 1), S1440–S1447. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10302777/#:~:text=The%20most%20common%20dietary%20sources,considered%20rich%20sources%20of%20betaine
- Conner, T. S., et al. (2018). Reducing occupational stress with a B-vitamin focussed intervention: A randomized clinical trial: Study protocol. Journal of Psychosomatic Research, 114, 41-49. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4290459/
- Hanifah, R., et al. (2018). B vitamins, work‐related stress and emotional mental disorders: A cross‐sectional study among nurses in Indonesia. Journal of Occupational Health, 60(1), 45– https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9190671/
- Gori, G., et al. (2021). Neurological symptoms of vitamin B12 deficiency: Analysis of pediatric patients. Journal of Pediatric Neurosciences, 16(3), 245–250. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6884369/
- Mason, J. B. (2021). Vitamin B6. In Encyclopedia of Human Nutrition. ScienceDirect. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/vitamin-b6
- Labrie, V., et al. (2020). Effects of negative stressors on DNA methylation in the brain: Implications for mood and anxiety disorders. Progress in Neurobiology, 187, 101778. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5096645/
- Zhang, Z., et al. (2022). Epigenetic alteration shaped by the environmental chemical bisphenol A. Frontiers in Endocrinology, 13, 847988. https://www.frontiersin.org/journals/genetics/articles/10.3389/fgene.2020.618966/full
- Adams, K. M., et al. (2023). Effect of aerobic/strength training on RANKL gene DNA methylation levels. Journal of Physical Activity and Health, 20(10), 755-763. https://journals.humankinetics.com/view/journals/jpah/20/10/article-p900.xml
- McClain, D. A., et al. (2022). Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Science Advances, 8(45), eabm5906. https://www.science.org/doi/10.1126/sciadv.aar8590
- National Center for Biotechnology Information. (n.d.). FOXP2 forkhead box P2 [Homo sapiens (human)]. Retrieved from https://www.ncbi.nlm.nih.gov/gene/93986#:~:text=FOXP2%20codes%20for%20a%20CNS,loss%20causes%20specific%20language%20impairment.&text=Truncation%20of%20FOXP2%20is%20the%20cause%20of%20developmental%20speech%20and%20language%20deficits




