Most Common Probiotics and Beneficial Gut Residents
Most Common Probiotics and Beneficial Gut Residents
What Are Probiotics?
Probiotics are live microorganisms that provide a health benefit to the host when they are consumed in adequate amounts.1 The word comes from the Greek term “pro bios” which means “for life.”2 These microorganisms add to the gut microbiome, potentially increasing the populations of beneficial gut residents that already reside there. Probiotics work by interacting with the anatomical, physical, and microbiological barriers of the gastrointestinal (GI) tract.3 Some of the proven benefits of probiotics include supporting healthy immune function and inflammation, reducing the risk of diseases including GI and allergic diseases, improving metabolic health, and acting as an antioxidant.1,3,4 Probiotics support the development and maintenance of a healthy microbiome through various mechanisms including:1-5
- Competitive exclusion of pathogens and inhibition of pathogen adhesion
- Production of antimicrobial substances
- Enhancing epithelial barrier function
- Modulating the immune system
- Modifying composition of gut microbiome
- Reducing colonic pH
- Generating metabolites, including butyrate and other short-chain fatty acids (SCFAs) that support gut health
A healthy intestinal tract, including a healthy gut microbiome, is important because it supports digestion as well as the immune system, metabolism, and cognitive systems.4 The gut is intricately connected to other parts of the body via axes to the brain, liver, bone, and even adipose tissue.6 Because of this, a healthy microbiome and probiotics can support distant functions.6
Prebiotics vs. Probiotics
Despite sounding similar, prebiotics differ significantly from probiotics. Probiotics are the actual live microbes that can colonize the GI tract while prebiotics are non-living components that feed gut bacteria. Prebiotics include fermentable dietary and functional fibers (inulin, fructo-oligosaccharides, and galacto-oligosaccharides) that cannot be digested by the body. Instead, they travel through the GI tract relatively unaltered, ultimately serving their purpose via selective use by bacteria in the gut microbiome, providing a dual health benefit to the bacteria and the host.1 When probiotics are combined with prebiotics, the product is considered a symbiotic; it provides live organisms (probiotics) and substrates to nourish them (prebiotics).1 Other “biotics” include:
- Psychobiotics
- Postbiotics
- Phytobiotics
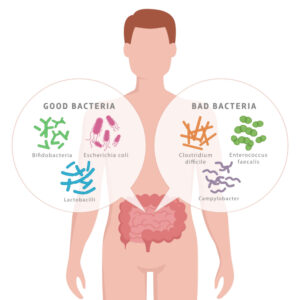
“Good” Vs. “Bad” Gut Microbes
Just as humans differ in terms of physical traits such as height or eye color, they also display unique gut microbiome profiles, making it difficult to precisely define a “healthy” microbiome. The composition of the gut microbiome in adults tends to be comprised primarily of Bacteroidetes and Firmicutes, with lower amounts of Actinobacteria, Proteobacteria, and Verucomicrobia.7,8 These are names of bacterial phyla (singular: phylum), which are grouped according to their unique morphological, physiological, metabolic, and ecological characteristics. Hierarchical classification after phylum includes class, order, family, genus, and species although it is common to refer to microbes by their genus and species only (for example Akkermansia muciniphila, or A. muciniphila). Everyday events can impact the abundance of the specific bacteria in the gut including exercise, dietary choices, infections, aging, and antibiotic use; but an individual’s general microbial composition tends to remain stable.8,9
A healthy microbiome will contain many different bacteria, but ultimately it is the proportion of “good” to “bad” microbes that will dictate the health of the GI tract and beyond. Commensal bacteria live in gut or body surfaces without harming human health, but they may cause issues when they spread to other parts of the body.10 An example of a commensal bacteria is Bacteroides fragilis, which is commonly found in the human colon but can cause infection if it gets into the bloodstream or other tissues. Mutualistic bacteria provide a benefit to the body and also receive a benefit from the host.10 For example, some bacteria help the body break down and digest food while also feeding off nutrients they encounter in the intestines. On the opposite end are pathogenic bacteria that can cause harm and disease to humans.10 The pathogenic bacteria Salmonella enterica and Campylobacter jejuni are commonly associated with foodborne illnesses. Altered composition, or dysbiosis of the gut, has been associated with various conditions including obesity, cardiovascular disease, diabetes, and neurodegenerative diseases.3,7
Just like food nourishes the body generally, it can also feed the gut specifically. Microbes that are fed well will flourish and colonize the GI tract, whether they are commensal, mutualistic, or pathogenic. Scientific studies have identified changes in specific bacteria populations due to dietary components including fibers, protein, phytochemicals, and a high fat diet.8 A high fiber, plant-based diet tends to feed “good” bacteria while a highly processed, Standard American Diet feeds the less helpful bacteria.8 Use of antibiotics, stress, or sickness can also alter the gut microbiome composition, possibly allowing pathogenic species to thrive when beneficial bacteria are depleted. But instead of having to rely solely on dietary choices to facilitate a healthy gut microbiome, it is possible to feed the gut with prebiotics and replenish the gut through targeted probiotics.
Common Types of Probiotics
Bifidobacterium
Members of the Bifidobacterium genus support the body by preventing pathogen infection via production of acetate, a short-chain fatty acid that can improve the health of the GI tract. Bifidobacteria levels in the gut are often noticeably altered in diseases states, including diabetes.8 A specific member of the Bifidobacterium genus, Bifidobacterium animalis subspecies lactis can exert beneficial effects on the body as a probiotic. It may positively affect stool frequency without increasing diarrhea and may modulate immune function including preventing upper respiratory tract infections, especially when combined with L. rhamnosus.5 B. lactis may also able to enhance immune health in elderly people.11
Prevotella
Levels of resident bacteria belonging to the Prevotella genus tend to be elevated in individuals that consume a plant-based diet and may contribute to improved glucose metabolism.12 Studies are ongoing to better understand the beneficial health effects of specific Prevotella species.12 Currently some studies indicate that Prevotella species may contribute to autoimmune disorders, gut inflammation, and insulin resistance although other studies indicate a protective effect.12 Additionally, the ratio of Prevotella to Bacteroides may be a useful marker of cardiometabolic health.12
Lactobacillus
Many probiotics belong to the group of lactic acid bacteria, including those in the Lactobacillus genus, which synthesize lactic acid through the fermentation of carbohydrates.4 The result is the secretion of secondary metabolites with important effects in the body including SCFAs, enzymes, biosurfactants, vitamins, plasmalogens, teichoic acids, and others.4 Probiotic intervention with various Lactobacilli strains were effective in both pre-clinical and clinical studies for addressing gastrointestinal diseases, skin conditions, metabolic disorders, and neurodegenerative diseases.13 L. acidophilus, L. paracasei, and L. rhamnosus are common probiotic species belonging to the Lactobacillus genus.
Lactobacillus acidophilus
L. acidophilus demonstrates anti-pathogenic activity and antioxidant activity when used in combination with other probiotic strains.4 When combined with the probiotic strain B. lactis, it resulted in stress reduction, reduced fat percentage, and decreased BMI and weight circumference.4 Similarly, in a clinical trial evaluating the effects of a yogurt containing L. acidophilus and B. lactis, consumption resulted in a reduction in triglycerides and cholesterol levels greater than the placebo group.4 L. acidophilus may provide benefits by altering levels of other microbes including increasing Akkermansia muciniphila, which has potent anti-obesity properties.14 Finally, it can also help reduce the frequency of diarrhea in children with acute gastroenteritis.15
Lacticaseibacillus paracasei
L. paracasei may be particularly suited to help with intestinal issues including diverticulitis, acute gastroenteritis, and diarrhea since it can survive passage through the GI tract, persists in stool after administration is stopped, and can temporarily colonize throughout different sites of the human colon.16 Other clinical benefits of L. paracasei as a probiotic include:16
- priming the systemic and mucosal immune response
- reducing fat storage and triglyceride levels
- enhancing immunity
- Reducing risk of infectious diseases and depression disorders
L. paracasei may also protect against the risk of upper respiratory tract infections in middle-age and elderly adults possibly by stimulating T cells of the immune system.3 Additionally, L. paracasei may help in recovery and improvement of muscle strength including benefiting blood markers of muscle injury and inflammation.17 Both live and heat-killed L. paracasei were beneficial in preventing strength loss after muscle damage, improving blood muscle damage and inflammatory markers, accelerating recovery, and lessening fatigue.17
Lacticaseibacillus rhamnosus
L. rhamnosus exhibits antioxidant, anti-pathogenic, and anti-inflammatory activity when used in combination with other Lactobacillus probiotics.4 It can also modify the immune response and inflammatory pathways.5 When combined with B. lactis, consumption in pregnant women resulted in a beneficial effect on blood glucose levels, insulin sensitivity, breast milk composition, and cytokine levels, indicating that combined supplementation with these two probiotic strains may help reduce the risk of gestational diabetes.5
Roseburia
Resident bacteria belonging to the Roseburia genus make up three to fifteen percent of a healthy microbiome and produce butyrate, which is likely the mechanism behind its beneficial effects. Decreased levels of Roseburia species have been associated with cardiovascular disease, inflammation, Parkinson’s disease, ulcerative colitis, and intestinal bowel disease.9,15 There is less clinical data available for Roseburia species and the exact role in human health is unclear, although studies are ongoing.9 One study showed that Roseburia hominis can improve intestinal barrier integrity and enhance anti-pathogenic activity, immune cell action, and anti-inflammatory actions.15
Faecalibacterium prausnitzii
F. prausnitzii belongs to the Firmicutes phylum and Faecalibacterium genus and is one of the most abundant butyrate-producing bacteria in the GI tract.18 It ferments glucose to produce butyrate, lactic acid, medium-chain fatty acids, and salicylic acid.2,9 Butyrate provides energy for intestinal epithelial cells, regulates intestinal T-cell activity, inhibits intestinal inflammation, and improves metabolic syndrome.9,18,19 Meanwhile, salicylic acid has anti-inflammatory effects and helps to maintain immune homeostasis in the body.9
When F. prausnitzii levels in the gut are depleted, dysbiosis can occur along with metabolic, gastrointestinal, and immune-related diseases including obesity, type 2 diabetes, and inflammatory diseases.9,15,18,19 Colonies of F. prausnitzii have been found to be lower in individuals with obesity and non-alcoholic fatty liver disease, but one study showed that three months of nutrition counseling successfully increased the quantity of F. prausnitzii.15,20 Enhancing F. prausnitzii abundance through dietary choices can stimulate the synthesis of mucin and tight junction proteins, enhancing intestinal mucosal barrier integrity.9 F. prausnitzii may serve as both a sensor and activator of intestinal health and can be used as a probiotic and prophylactic treatment to avoid immune-related inflammatory diseases.2,8
Akkermansia muciniphila
As a member of the Akkermansia genus in the Verrucomicrobia phylum, A. muciniphila comprises one to five percent of the bacteria in the gut microbiome in healthy individuals.2,8,9 Lower levels of A. muciniphila have been found in patients with obesity, type 2 diabetes, irritable bowel disease, high blood pressure, and liver diseases.2,6,9 It can pass from mother to newborn through breast milk, which explains its presence in the digestive tract of newborn infants.2 Dietary factors that can increase the abundance of A. muciniphila include polyphenols, caloric restriction, and fructo-oligosaccharides.6
The presence of A. muciniphila in the gut can enhance the host’s immune system, metabolic processes, and gut health.2 It possesses beneficial activity against obesity, diabetes, metabolic syndrome, neurodegenerative diseases, cancer, inflammation, and aging.6 Additionally, A. muciniphila produces the SCFAs propionate and acetate which can help improve intestinal barrier function and intestinal health.2 It can also control immunological function in the gut.2 In a pre-clinical model, A. muciniphila reversed obesity induced by high fat diet, reduced insulin resistance and heart complications, and helped stop the release of and passage of metabolic endotoxins.9,15 However, some studies have found that increased abundance is associated with aggravated disease progression including colitis and intestinal inflammation.6 Preliminary findings indicate it is safe but human clinical trials will be able to clarify its safety and efficacy.2
Yeast Probiotics
The gut microbiome is more than bacteria; it is also made up of fungi, viruses, and protozoa.3 Fungi make up approximately 0.1 percent of total gut microbes, but they play an important role in intestinal homeostasis and human health, affecting the development of autoimmune, metabolic, and neurological disorders and cancer.21 The fungi of the gut microbiome, also called the mycobiome, demonstrates higher variability between individuals and greater instability over time compared to the bacteria of the microbiome.21
Probiotic yeasts produce active metabolites including mycocins, volatile organic compounds, enzymes, and peptides, inhibit the growth of pathogens, stimulate the immune system, and improve gut health. They can also survive acidic conditions during digestion, enabling them to reach the intestines where they can adhere to human intestinal cells.22 Probiotic yeast may be a safer option than some probiotics due to their lack of ability to transfer resistant genes to pathogenic bacteria, which is a risk when utilizing antibiotic resistant probiotic strains such as Lactobacillus.22
Pichia
Yeast species belonging to the Pichia genus, including Pichia kudriavzevii, Pichia fermentans, and Pichia guilliermondii, work as probiotics in the gut.23 Several Pichia strains or their metabolites have been found benefit the gut, reduce cholesterol levels, and induce cancer cell death.23,24
Saccharomyces cerevisiae
Saccharomyces cerevisiae, also called brewer’s or baker’s yeast, of the Ascomycota phylum can exert beneficial effects on the body.25 It is the most commonly used yeast in food fermentation, but it also functions as a probiotic in the body.25 S. cerevisiae can help inhibit bacterial pathogens, reduce pro-inflammatory cytokines in the gut, and activate both the innate and adaptive immune systems of the host during a pathogen infection.25 It is important in maintaining and restoring intestinal homeostasis and helps protect against oxidative damage.25 S. cerevisiae can also produce folate, an essential B vitamin.25 Clinical studies in humans demonstrated its effectiveness for various GI diseases including antibiotic-associated diarrhea and ulcerative colitis; however, there are possible interactions and side effects associated with S. cerevisiae.25
Saccharomyces boulardii
Saccharomyces cerevisiae var. boulardii is the most studied and widely available probiotic yeast species.22,26 It was originally thought to be a different species than S. cerevisiae, but it currently has been categorized as a variety of S. cerevisiae.23 It is primarily used for treating GI diseases and utilized for its natural ability to resist antibiotic treatment.22,26 It can also produce bioactive compounds, including the antioxidant glutathione, and can stimulate activity of immune cells.3,26
In clinical trials, treatment with S. boulardii resulted in improvements in gastrointestinal diseases including multiple forms of diarrhea, C. diff-associated syndrome, ulcerative colitis, irritable bowel syndrome, and Crohn’s disease.23,26 It is also being studied for its ability to inhibit cancer cell development and progression, enhance growth of skin, hair, and nails, increase glucose sensitivity, and protect from bacterial infections.26
Considerations
Mechanisms underlying the effects of probiotics are difficult to fully elucidate and scientific studies seeking to understand health effects of probiotics are challenging. For example, some bacterial strains are extremely sensitive to oxygen levels, making them difficult to study, not to mention deliver to the body in a supplement.9 Probiotics also exhibit strain-specific effects and administering only a single strain may have different results than when multiple strains are combined.1,3 Additionally, approximately 80 percent of intestinal bacteria are unknown, so it is possible more changes are occurring than can be detected at this point.9
Clinical trials investigating probiotics are complicated by the many dietary compounds that can modulate the gut microbiota include fibers acting as prebiotics and omega-3 fatty acids which can enhance the production of anti-inflammatory short-chain fatty acids.7,8 It can be difficult to control or account for these effects when trying to pinpoint specific functions of a single probiotic. So, while one compound may negatively affect a bacterial population, another may support it, masking any effect a probiotic is having on the population. There is also likely variation between individuals due to their age, sex, or health status which can result in better or worse results with a given probiotic.9 Clinical studies investigating the effects of probiotics are increasing, but much of the mechanistic work utilizes in vitro and in vivo models, limiting translatability to humans until results can be confirmed.4
Reviewed by Weston Bussler, PhD
- Fagnant, H.S., Isidean, S.D., Wilson, L., Bukhari, A.S., Allen, J.T., Agans, R.T., Lee, D.M., Hatch-McChesney, A., Whitney, C.C., Sullo, E., Porter, C.K., Karl, J.P. (2023). Orally Ingested Probiotic, Prebiotic, and Synbiotic Interventions as Countermeasures for Gastrointestinal Tract Infections in Nonelderly Adults: A Systematic Review and Meta-Analysis. Adv Nutr, S2161:135.
- Effendi, R.M.R.A., Anshory, M., Kalim, H., Dwiyana, R.F., Suwarsa, O., Pardo, L.M., Nijsten, T.E.C., Thio, H.B. (2022). Akkermansia muciniphila and Faecalibacterium prausnitzii in Immune-Related Diseases. Microorganisms, 10:2382.
- Szydlowska, A., Sionek, B. (2023). Probiotics and Postbiotics as the Functional Food Components Affecting the Immune Response. Microorganisms, 11:104.
- Vera-Santander, V.E., Hernandez-Figueroa, R.H., Jimenez-Munguia, M.T., Mani-Lopez, E., Lopez-Malo, A. (2023). Health Benefits of Consuming Foods with Bacterial Probiotics, Postbiotics, and Their Metabolites: A Review. Molecules, 28:1230.
- Flach, J., van der Waal, M.B., Kardinaal, A.F.M., Schloesser, J., Ruijschop, R.M.A.J., Claassen, E. (2018). Probiotic research priorities for the healthy adult population: A review on the health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12.
- Jian, H., Liu, Y., Wang, X., Dong, X., Zou, X. (2023). Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut-Liver-Brain Axes? Int J Mol Sci, 24:3900.
- Jagielski, P., Boleslawska, I., Wybranska, I., Przyslawski, J., Luszczki, E. (2023). Effects of a Diet Containing Sources of Prebiotics and Probiotics and Modification of the Gut Microbiota on the Reduction of Body Fat. Int J Environ Res Public Health, 20:1348.
- Ganesan, K., Chung, S.K., Vanamala, J., Xu, B. (2018). Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int J Mol Sci, 19:3720.
- Zhang, H., Duan, Y., Cai, F., Cao, D., Wang, L., Qiao, Z., Hong, Q., Li, N., Zheng, Y., Su, M., Liu, Z., Zhu, B. (2022). Next-Generation Probiotics: Microflora Intervention to Human Diseases. Biomed Res Int, 2022:5633403.
- Eloe-Fadrosh, E.A., Rasko, D.A. (2013). The Human Microbiome: From Symbiosis to Pathogenesis. Annu Rev Med, 64:145.
- Arunachalam, K., Gill, H.S., Chandra, R.K. (2000). Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur J Clin Nutr, 54(3):263.
- Huang, F., Sardari, R.R.R., Jasilionis, A., Book, O., Oste, R., Rascon, A., Heyman-Linden, L., Holst, O., Karlsson, E.N. (2020). Cultivation of the gut bacterium Prevotella copri DSM 18305T using glucose and xylose as carbon sources. Microbiologyopen, 10(3):e1213.
- Un-Nisa, A., Khan, A., Zakria, M., Siraj, S., Ullah, S., Tipu, M.K., Ikram, M., Kim, M.O. (2023). Updated on the Role of Probiotics against Different Health Issues: Focus on Lactobacillus. Int J Mol Sci, 24:142.
- Ondee, T., Pongpirul, K., Visitchanakun, P., Saisorn, W., Kanacharoen, S., Wongsaroj, L., Kullapanich, C., Ngamwongsatit, N., Settachaimongkon, S., Somboonna, N., Leelahavanichkul, A. (2021). Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci Rep, 11:6367.
- Cheng, H.-L., Yen, G.-C., Huang, S.-C., Chen, Hsu, C.-L. (2022). The next generation beneficial actions of novel probiotics as potential therapeutic targets and prediction tool for metabolic diseases. J Food Drug Anal, 30(1):1.
- Jones, R.M. (2017). The Use of Lactobacillus casei and Lactobacillus paracasei in Clinical Trials for the Improvement of Human Health. The Microbiota in Gastrointestinal Pathophysiology, 99-107.
- Lee, M.-C., Ho, C.-S., Hsu, Y.-J., Huang, C.-C. (2022). Live and Heat-Killed Probiotic Lactobacillus paracasei PS23 Accelerated the Improvement and Recovery of Strength and Damage Biomarkers after Exercise-Induced Muscle Damage. Nutrients, 14:4563.
- Bai, Z., Zhang, N., Jin, Y., Chen, L., Mao, Y., Sun, L., Fang, F., Liu, Y., Han, M., Li, G. (2023). Comprehensive analysis of 84 Faecalibacterium prausnitzii strains uncovers their genetic diversity, functional characteristics, and potential risk. Front Cell Infect Microbiol, 12:919701.
- Maioli, T.U., Borras-Nogues, E., Torres, L., Barbosa, S.C., Martins, V.D., Langella, P., Azevedo, V.A., Chatel, J.-M. (2021). Possible Benefits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front Pharmacol, 12:740636.
- Jin, L., Xu, M.-Z. (2023). Characterization of gut dominant microbiota in obese patients with nonalcoholic fatty liver disease. Front Cell Infect Microbiol, 13:1113643.
- Zhang, F., Aschenbrenner, D., Yoo, J.Y., Zuo, T. (2022). The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe, 3(12):e969.
- Shruthi, B., Deepa, N., Somashekaraiah, R., Adithi, G., Divyashree, S., Sreenivasa, M.Y. (2022). Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol Rep (Amst), 34:e00716.
- Staniszewski, A., Kordowska-Wiater, M. (2021). Probiotic and Potentially Probiotic Yeasts- Characteristics and Food Application. Foods, 10:1306.
- Saber, A., Alipour, B., Faghfoori, Z., Jam, A.M., Khosroushahi, A.Y. (2017). Secretion metabolites of probiotic yeast, Pichia kudriavzevii AS-12, induces apoptosis pathways in human colorectal cancer cell lines. Nutr Res, 41:36.
- Farid, F., Sideeq, O., Khan, F., Niaz, K. (2019). Chapter 5.1- Saccharomyces cerevisiae. Nonvitamin and Nonmineral Nutritional Supplements,
- Abid, R., Waseem, H., Ali, J., Ghazanfar, S., Ali, G.M., Elasbali, A.M., Alharethi, S.H. (2022). Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. Fungi, 8:444.